
Concept explainers
Draw the products formed when
a.
b.
c.
d.
e.
f.
g.
h.
![Chapter 11, Problem 11.33P, 11.33 Draw the products formed when is treated with each reagent.
[2] ;](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780078021558/Chapter-11/images/21558-11-11.33p-question-digital_image001.jpg) ;
;
(a)
Interpretation: The product that is formed by the reaction of
Concept introduction: The addition of an electrophile to an alkyne is followed by Markovnikoff’s rule and anti-stereoselectivity.
The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of bromine atoms to the alkyne chain is known as bromination. Bromination can be done by using the reagents like
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
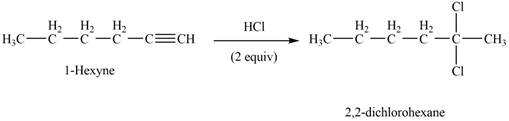
Figure 1
The reaction of
The product that is formed by the reaction of
(b)
Interpretation: The product that is formed by the reaction of
Concept introduction: The addition of an electrophile to an alkyne is followed by Markovnikoff’s rule and anti-stereoselectivity.
The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of bromine atoms to the alkyne chain is known as bromination. Bromination can be done by using the reagents like
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
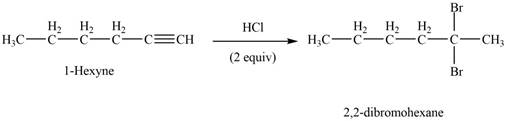
Figure 2
The reaction of
The product that is formed by the reaction of
(c)
Interpretation: The product that is formed by the reaction of
Concept introduction: The addition of an electrophile to an alkyne is followed by Markovnikoff’s rule and anti-stereoselectivity.
The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of bromine atoms to the alkyne chain is known as bromination. Bromination can be done by using the reagents like
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
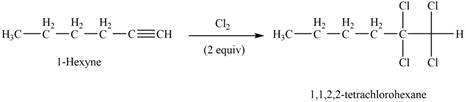
Figure 3
The reaction of
The product that is formed by the reaction of
(d)
Interpretation: The product that is formed by the reaction of
Concept introduction: A terminal alkyne reacts with
The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms.
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
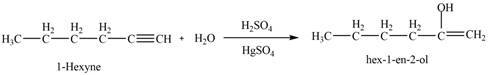
Figure 4
The reaction of
Then, the enol form,
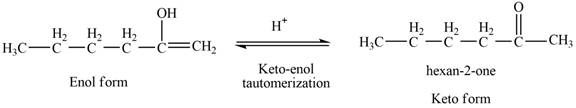
Figure 5
Thus, the keto-enol tautomerization of
The product that is formed by the reaction of
(e)
Interpretation: The product that is formed by the reaction of
Concept introduction: A stepwise procedure of transforming an alkyne into a carbonyl group is known hydroboration-oxidation reaction. In a hydroboration-oxidation reaction, a terminal alkyne reacts with
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
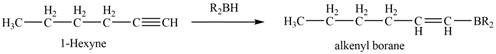
Figure 6
The reaction of
Then, the reaction of alkenylborane with
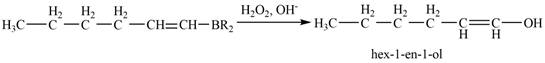
Figure 7
Thus, the reaction of alkenylborane with
In the last step,
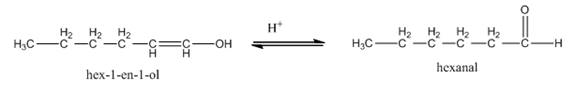
Figure 8
Thus, the tautomerization of
The product that is formed by the reaction of
(f)
Interpretation: The product that is formed by the reaction of
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
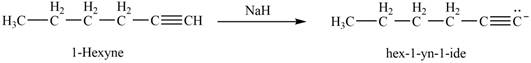
Figure 9
The reaction of
(a) The product that is formed by the reaction of
(g)
Interpretation: The product that is formed by the reaction of
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.33P
The product that is formed by the reaction of
Explanation of Solution
The reaction of
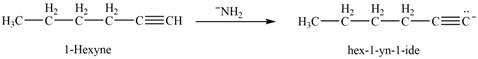
Figure 10
The reaction of
Then, the reaction of
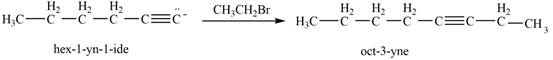
Figure 11
Thus, in the above reaction,
The product that is formed by the reaction of
(h)
Interpretation: The product that is formed by the reaction of ![]() ;
;
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.33P
The product that is formed by the reaction of ![]() ;
;
Explanation of Solution
The reaction of
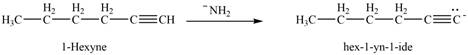
Figure 12
The reaction of
Then, the reaction of
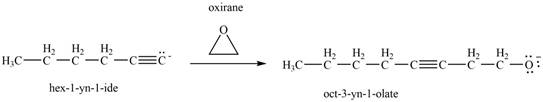
Figure 13
Thus, in the above reaction,
Then, the reaction of
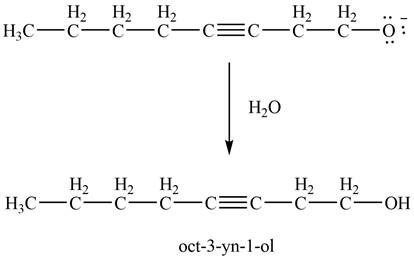
Figure 14
Thus, in the above reaction,
The product that is formed by the reaction of ![]() ;
;
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry (7th Edition)
Organic Chemistry
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: Structure and Properties
Essential Organic Chemistry (3rd Edition)
- #20 B Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HCl.arrow_forwardDraw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl.arrow_forwardWhat product is formed when CH3OCH2CH2C ≡ CCH2CH(CH3)2 is treated with each reagent: (a) H2 (excess), Pd-C; (b) H2(1 equiv), Lindlar catalyst; (c) H2 (excess), Lindlar catalyst; (d) Na, NH3?arrow_forward
- Draw the products formed when hex-1-yne is treated with each reagent. a. HCl (2 equiv) b. HBr (2 equiv) c. Cl2 (2 equiv) d.H2O + H2SO4 + HgSO4 e. [1] R2BH; [2] H2O2, HO− f. NaH g. [1] −NH2; [2] CH3CH2Brarrow_forwardWhat would be the reagent for each step?arrow_forwardDraw the organic product(s) formed when CH3CH2CH2OH is treated with each reagent. a.H2SO4 b.NaH c.HCl + ZnCl2 d.HBr e.SOCl2, pyridine f.PBr3 g.TsCl, pyridine h. [1] NaH; [2] CH3CH2Br [1] i.TsCl, pyridine; [2] NaSH j.POCl3, pyridinearrow_forward
- What reagent can be used from compound F to G? (NaH / NaOH / LiAlH4+hydronium quench / CrO3 Jones)arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs.a. NaBH4, CH3OHb. [1] LiAlH4; [2] H2Oc. [1] CH3MgBr (excess); [2] H2Od. [1] C6H5Li (excess); [2] H2Oe. Na2Cr2O7, H2SO4, H2Oarrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent (d, e and f)arrow_forward
- Draw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent. a. HCl b. CH3COCl c. (CH3CO)2O d. excess CH3I e. (CH3)2C = O f. CH3COCl, AlCl3 g. CH3CO2H h. NaNO2, HCl i. Part (b), then CH3COCl, AlCl j. CH3CHO, NaBH3CNarrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent (a, b and c)arrow_forwardWhat are the two missing reagents?arrow_forward
