
Concept explainers
Predict the major products of the following reactions, including stereochemistry where appropriate.
- a. (R)-butan-2-ol+TsCI In pyridine
- b. (S)-2-butyl tosylate+NaBr
- c. cyclooctanol+NaOCI/HOAc
- d. cyclopentylmethanol+CrO3·pyridine·HCl
- e. cyclopentylmethanol+Na2Cr207/H2SO4
- f. cyclopentanol+HCl/ZnCl2
- g. n-butanol+HBr
- h. cyclooctylmethanol+CH3CH2MgBr
- i. potassium tert-butoxide+methyliodide
- j. sodium methoxide+tert-butyliodide
- k. cyclopentanol+H2SO4/heat
- l. product from (k)+OsO4/H2O2, then HIO4
- m. sodiumethoxide+1-bromobutane
- n. sodiumethoxide+2-methyl-2-bromobutane
- ○. octan-1-ol + DMSO + oxalyl chloride
- p. 4-cyclopentylhexan-1-ol + DMP reagent
(a)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Tosyl chloride in pyridine as a reagent is used to convert alcohols into respective tosylate esters through retention of configuration.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 1.
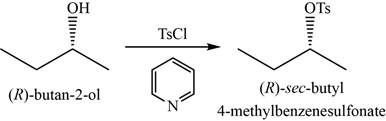
Figure 1
(b)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium bromide is used to convert the compounds of tosylate ester into respective bromide through inversion of configuration.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 2.
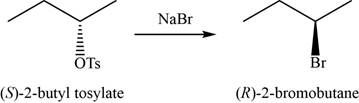
Figure 2
(c)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium hypochlorite with acetic acid
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between cyclooctanol and
The product of the given reaction is shown in Figure 3.
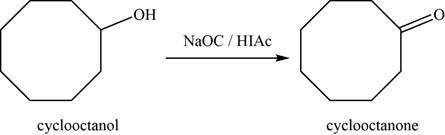
Figure 3
(d)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Jones reagent
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentanecarbaldehyde.
Explanation of Solution
The given reaction occurs between cyclopentylmethanol and
The product of the given reaction is shown in Figure 4.
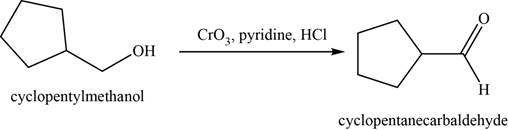
Figure 4
(e)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Jones reagent
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentanecarboxylic acid.
Explanation of Solution
The given reaction occurs between cyclopentylmethanol and
The product of the given reaction is shown in Figure 5.
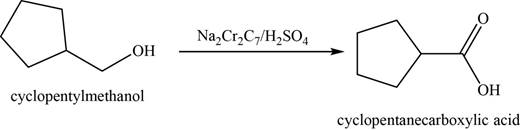
Figure 5
(f)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Lucas reagent
Answer to Problem 11.39SP
The major product of the given reaction is chlorocyclopentane.
Explanation of Solution
The given reaction occurs between cyclopentanol and
The product of the given reaction is shown in Figure 6.
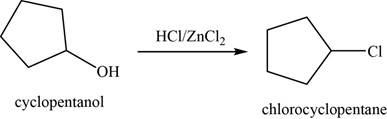
Figure 6
(g)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The halo acids like
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 7.
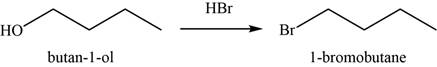
Figure 7
(h)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The reaction of alcohols with Grignard reagent leads to the formation of adduct complex of Grignard.
Answer to Problem 11.39SP
The major product of the given reaction is magnesium bromide cyclooctylmethanolate.
Explanation of Solution
The given reaction occurs between cyclooctylmethanol and
The product of the given reaction is shown in Figure 8.
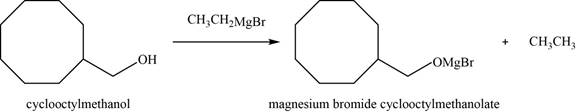
Figure 8
(i)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Methyl iodide is used to convert an alkoxide into ether with removal of halo-salts.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between potassium tert-butoxide and methyliodide. Methyl iodide is used to convert an alkoxide into ether with removal of halogen salts.
The product of the given reaction is shown in Figure 9.
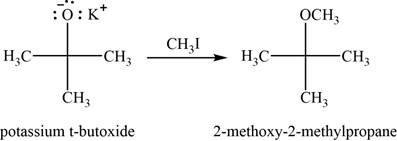
Figure 9
(j)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium methoxide is a strong base, used for the conversion of bulkier haloalkane into an alkene with removal of an alcohol.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium methoxide and tert-butyliodide. Sodium methoxide is a strong base, used for the conversion of bulkier haloalkane into an alkene with the removal of an alcohol.
The product of the given reaction is shown in Figure 10.
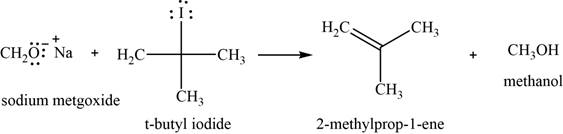
Figure 10
(k)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The sulphuric acid catalyzed reaction is used to convert alcohols into an alkene through dehydration and
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentene.
Explanation of Solution
The given reaction occurs between cyclopentanol and
The sulphuric acid catalyzed reaction is used to convert alcohols into an alkene through dehydration and
The product of the given reaction is shown in Figure 11.
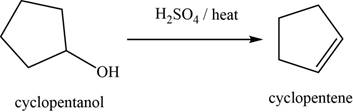
Figure 11
(l)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Osmium tetra oxide
Answer to Problem 11.39SP
The major product of the given reaction is glutaraldehyde.
Explanation of Solution
The given reaction occurs between cyclopentene (product from k) and
The product of the given reaction is shown in Figure 12.
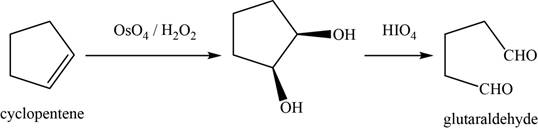
Figure 12
(m)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium ethoxide is a strong base and used to convert haloalkanes into respective ether through
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium ethoxide and
Sodium ethoxide is a strong base and used to convert haloalkanes into respective ether through
The product of the given reaction is shown in Figure 13.
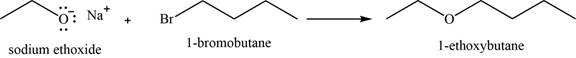
Figure 13
(n)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium ethoxide is a strong base and used to convert bulkier haloalkanes into respective alkenes.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium ethoxide and
Sodium ethoxide is a strong base and used to convert bulkier haloalkanes into respective alkenes.
The product of the given reaction is shown in Figure 14.
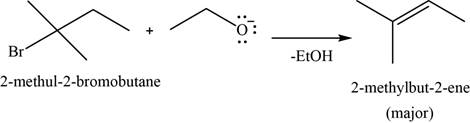
Figure 14
(o)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Swern oxidation of alcohols to convert it into aldehydes are done by
Answer to Problem 11.39SP
The major product of the given reaction is octanal.
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 15.
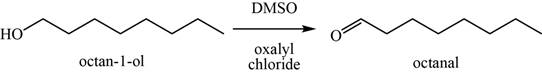
Figure 15
(p)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 16.
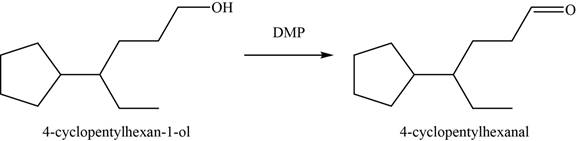
Figure 16
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry (9th Edition)
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry: Structure and Properties
Living By Chemistry: First Edition Textbook
CHEMISTRY-TEXT
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry & Chemical Reactivity
- Choose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forward2) Draw and name the organic compound found in every reaction. d) Reaction of cis-3,3-Dimethyl-4-propylocta-1,5-diene with two mole of HBr e) Reaction of trans-1-Bromo-3-chlorocyclopentane with potassium hydroxide f) Formation of Gilman reagent using isopropyl bromide g) Ozonolysis of 3,3-Dimethyloct-4-yne h) Complete halogenation (Cl2) of 3-Ethyl-5-methyl-1,6,8-decatriyne i) Partial hydrogenation using Lindlar's Catalyst 2,2,5,5-Tetramethylhex-3-yne j) Reaction of 3,4-Dimethylcyclodecyne with sodium amidearrow_forwardPredict the major products of the following reactions.(a) 1@ethylcycloheptene + ozone, then (CH3)2S(b) 1@ethylcycloheptene + warm, concentrated KMnO4(c) 1@ethylcycloheptene + cold, dilute KMnO4arrow_forward
- When 3-methyl-1-butene is reacted with 9-borabicyclo[3.3.1]nonane, the "1-ol" product is formed. What is the detailed reactin scheme for the transformation? Describe the purification procedure.arrow_forwardPredict the product of the following reaction and classify the reaction. Pb(NO3)2+FeSO4--->PbSO4+________arrow_forwardOrganotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or fungicides.One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive trialkylaluminum compounds, such as liquid triethylaluminum (Al(C2H5)3). 3SnCl4 + 4Al(C2H5)3 3Sn(C2H5)4 + 4AlCl3 In one experiment, 0.230 L of SnCl4 (d = 2.226 g/mL) was treated with 0.396 L of triethylaluminum (Al(C2H5)3); d = 0.835 g/mL). If 0.335 L of tetraethylstannane (d = 1.187 g/mL) were actually isolated in this experiment, what was the percent yield?arrow_forward
- Predict the products of the reactions of the following compounds with:(1) chromic acid or excess sodium hypochlorite with acetic acid.(2) PCC or NaOCl (1 equivalent) with TEMPO. (a) cyclohexane (b) 1-phenylpropan-1-ol (c) hexan-1-ol (d) acetaldehyde, CH3CHOarrow_forwardChoose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) KMnO4, H3O+ CH3Cl, AlCl3 HNO3, H2SO4 Cl2, FeCl3 fuming sulfuric acidarrow_forwardGiven: Mass spec for 4-acetylbiphenyl. Knowing that 196 m/z is the major product, how would you assign 181 m/z and 152 m/z? (i've also attached a picture of the reaction scheme)arrow_forward
- give the reagents for parts a-parrow_forwardOrganotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or fungicides. One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive trialkylaluminum compounds, such as liquid triethylaluminum (Al(C2H5)3). 3SnCl4 + 4AI(C2H5)3 →3Sn(C2H5)4 + 4AlCl3 In one experiment, 0.160 L of SnCl4 (d = 2.226 g/mL) was treated with 0.346 L of triethylaluminum (Al(C2H5)3): d = 0.835 g/mL). What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C2H5)4)? If 0.257 L of tetraethylstannane (d= 1.187 g/mL) were actually isolated in this experiment, what was the percent yield?arrow_forwardWhen Br2 is added to buta-1,3-diene at -15 °C, the product mixture contains 60% ofproduct A and 40% of product B. When the same reaction takes place at 60 °C, theproduct ratio is 10% A and 90% B.(a) Propose structures for products A and B. (Hint: In many cases, an allylic carbocationis more stable than a bromonium ion.)arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


