
Concept explainers
The following names are incorrect, according to IUPAC rules. Draw the structural formulas and tell why each name is incorrect. Write the correct name for each compound.
a.
b.
c.
d.
(a)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
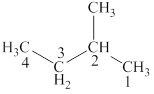
The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
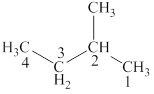
Figure 1
The structural formulas for given compound is shown in Figure 1. The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
(b)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
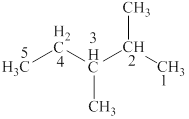
The lowest possible number is given to the carbon at which a group is attached. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is

Figure 2
The structural formulas for given compound is shown in Figure 2. The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is
(c)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
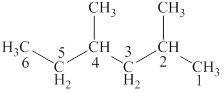
The parent chain is hexane and one methyl group each is attached to second and fourth carbon atom. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has six carbon atoms. Thus, the parent chain is hexane. The lowest possible number is given to the carbon from which a group is attached. Thus, methyl group are attached to second and fourth carbon. Thus, the correct IUPAC name of the given compound is

Figure 3
The structural formulas for given compound is shown in Figure 3. The parent chain is hexane and one methyl group each is attached to second and fourth carbon atoms. Thus, the correct IUPAC name of the given compound is
(d)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
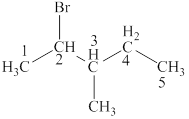
The parent chain is pentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has five carbon atoms. Thus, the parent chain is pentane. The lowest possible number is given to the carbon from which a group is attached. Thus, bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is

Figure 4
The structural formulas for given compound is shown in Figure 4. The parent chain ispentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- The correct structural for ethyne is: a. HC=CH b. HC=C=H c. HCCH d. H=C=C=Harrow_forwardThe compound CH2=CHCH2CH2CH3 is an example of: a. a pentane. b. a hexene. c. an alkene. d. organic macromolecule.arrow_forwardDraw the structural formula for each of the following. a. 3-isobutylhexane b. 2,2,4-trimethylpentane, also called isooctane. This substance is the reference (100 level) for octane ratings. c. 2-tert-butylpentane d. The names given in parts a and c are incorrect. Give the correct names for these hydrocarbons.arrow_forward
- Given this information, match the structural formula of a compound with an unbranched chain of four carbon atoms that is an:(a) alkanone (b) alkanoic acid (c) alkene (d) alkynol (e) alkanamine (f) alkanalUnder each structural formula, write the matching compound name from parts (a) through (f). Example: alkane.arrow_forwarda. Which of the following compounds have the same empirical formula?(1) But-2-ene(2) Propane(3) EtheneA. (1) and (2) onlyB. (1) and (3) onlyC. (2) and (3) onlyD. (1), (2) and (3) b. Which of the following statements concerning homologous series is INCORRECT?A. Members of a series have identical chemical properties.B. Members of a series have the same general formula.C. Each member differs from the next by a −CH2− group.D. Members of a series show a gradual change of physical properties.arrow_forwardWhat kinds of reactions are common to alkanes? List an example of each.arrow_forward
- Consider the reaction in the attached picture: 1. The reaction is an example of ______. a. Addition reaction b. substitution reaction c. elimination reaction 2. The reaction shows _________ a. Oxidation of alcohol to produce an alkene b. Dehydration of alcohol to produce an alkyne c. Dehydration of alcohol to produce an alkenearrow_forwardDraw the structural formula for each of the following. a.3-isobutylhexane b.2,2,4-trimethylpentane, also called isoocta11e. This substance is the reference (100 level) for octane ratings. c. 2-tert-butylpentane d.The names given in parts a and c are incorrect.Give the correct names for these hydrocarbonsarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





