
Interpretation: Given the decomposition of
Concept Introduction:
The integrated rate law equation explains how the concentrations of reactants change with time.
Consider a first order
The concentration of the reactant A at time t is given by the below equation
Where,
The integrated rate law for this first order reaction is obtained by taking the natural logarithm of both sides of
That is,
Using Dalton's law, the partial pressure of formic acid is given by
Where,
The order of the reaction can be determined from a plot of concentration against time.
If we plot concentration against time, and if the curve is linear, the reaction is a zero order reaction.
If we plot log of concentration against time and if the curve is linear, the reaction is a first order reaction.
If we plot concentration inverse against time and if the curve is linear, the reaction is a second order reaction.
Answer to Problem 11.50PAE
Solution: The rate constant is
Explanation of Solution
Given Information: The table containing the total pressures in the reaction vessel during the decomposition of
Decomposition of
The initial partial pressure of
Calculate the partial pressure of
Partial pressure of
Partial pressure of
Partial pressure of
Therefore, the total pressure at any given time t is given as
| Time(t) | Total pressure |
|
| 0 | 491.7 | 491.7 |
| 185.3 | 549.6 | 434.0 |
| 242.8 | 566.6 | 417.0 |
| 304.5 | 584.1 | 399.5 |
| 362.7 | 599.9 | 383.7 |
| 429.5 | 617.2 | 366.4 |
| 509.7 | 637.0 | 346.6 |
| 606.3 | 659.5 | 324.1 |
We need to plot these values of partial pressure with time to see if the reaction is zero order or not
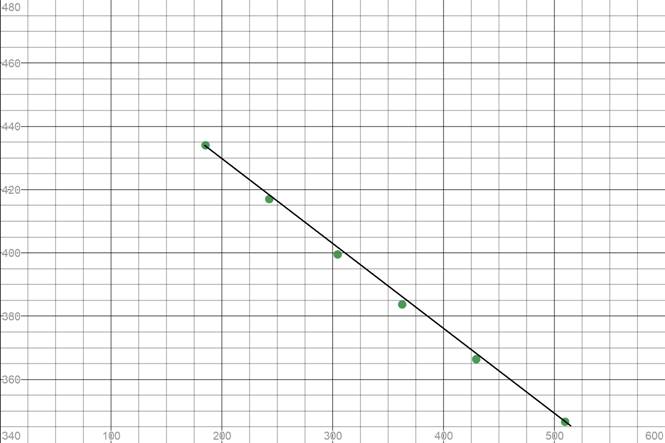
As some points do not lie on the straight line, the curve is not linear. Thus, it is not a zero order reaction.
| Time(t) | |
|
| 0 | 491.7 | 6.1978687744 |
| 185.3 | 434.0 | 6.0730445341 |
| 242.8 | 417.0 | 6.0330862218 |
| 304.5 | 399.5 | 5.9902137652 |
| 362.7 | 383.7 | 5.9498609973 |
| 429.5 | 366.4 | 5.9037256328 |
| 509.7 | 346.6 | 5.8481713773 |
| 606.3 | 324.1 | 5.7810521101 |
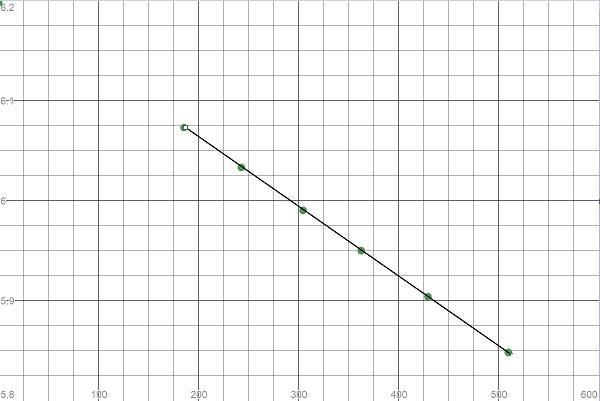
Here we see the curve is linear and thus the reaction is a first order reaction.
To calculate the rate constant, we need the negative slope of the line in the plot
Hence, the rate constant is the negative of the slope obtained. It is equal to
The concept of integrated rate law and the manipulation the data into a plot helps in determining the order of the decomposition of
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Engineering Students
- At 40C, H2O2 (aq) will decompose according to the following reaction: 2H2O2(aq)2H2O(l)+O2(g) The following data were collected for the concentration of H2O2 at various times. Times(s) [H2O2](mol/L) 0 1.000 2.16 104 0.500 4.32 104 0.250 a. Calculate the average rate of decomposition of H2O2 between 0 and 2.16 104 s. Use this rate to calculate the average rate of production of O2(g) over the same time period. b. What are these rates for the time period 2.16 104 s to 4.32 104 s?arrow_forwardPhenyl acetate, an ester, reacts with water according to the equation The data in the table were collected for this reaction at 5 C. (a) Plot the phenyl acetate concentration versus time, and describe the shape of the curve observed. (b) Calculate the rate of change of the phenyl acetate concentration during the period 15.0 seconds to 30.0 seconds and also during the period 75.0 seconds to 90.0 seconds. Why is one value smaller than the other?arrow_forwardThe initial rate for a reaction is equal to the slope of the tangent line at t 0 in a plot of [A] versus time. From calculus, initial rate = d[A]dt . Therefore. the differential rate law for a reaction is Rate = d[A]dt=k[A]n. Assuming you have some calculus in your background, derive the zero-, first-, and second-order integrated rate laws using the differential rate law.arrow_forward
- Sulfuryl chloride (SO2Cl2) decomposes to sulfur dioxide (SO2) and chlorine (Cl2) by reaction in the gas phase. The following pressure data were obtained when a sample containing 5.00 102 mol sulfuryl chloride was heated to 600. K in a 5.00 101-L container. Time (hours): 0.00 1.00 2.00 4.00 8.00 16.00 PSO2Cl2(atm): 4.93 4.26 3.52 2.53 1.30 0.34 Defining the rate as [SO2Cl2]t, a. determine the value of the rate constant for the decomposition of sulfuryl chloride at 600. K. b. what is the half-life of the reaction? c. what fraction of the sulfuryl chloride remains after 20.0 h?arrow_forwardA study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a) Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s. (b) Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate? (c) Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).arrow_forwardThe thermal decomposition of diacetylene, C4H2, was studied at 950 C. Use the following data (K. C. Hou and H. B. Palmer, Journal of Physical Chemistry. Vol. 60, p. 858, 1965) to determine the order of the reaction.arrow_forward
- Experimental data are listed here for the reaction B: Time (s) IB] (mol/L) 0.00 0.000 10.0 0.326 20.0 0.572 30.0 0.750 40.0 0.890 Prepare a graph from these data, connect the points with a smooth line, and calculate the rate of change of [B] for each 10-s interval from 0.0 to 40.0 s. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result. How is the rate of change of [AJ related to the rate of change of [B] in each time interval? Calculate the rate of change of [AJ for the time interval from 10.0 to 20.0 s. What is the instantaneous rate, A[B]/Ar, when [BI = 0.750 mol/L?arrow_forwardThe decomposition of ozone is a second-order reaction with a rate constant of 30.6 atm1 s1 at 95 C. 2O3(g)3O2(g) If ozone is originally present at a partial pressure of 21 torr, calculate the length of time needed for the ozone pressure to decrease to 1.0 torr.arrow_forward6. Phenyl acetate, an ester, reacts with water according to the equation The data in the table were collected for this reaction at 5°C. Time (s) [Phenyl acetate] (mol/L) 0 0.55 15.0 0.42 30.0 0.31 45.0 0.23 60.0 0.17 75.0 0.12 90.0 0.085 Plot the phenyl acetate concentration versus time, and describe the shape of the curve observed. Calculate the rate of change of the phenyl acetate concentration during the period 15.0 seconds to 30.0 seconds and also during the period 75.0 seconds to 90.0 seconds. Why is one value smaller than the other? What is the rate of change of the phenyl acetate concentration during the time period 60.0 seconds to 75.0 seconds? What is the instantaneous rate at 15.0 seconds?arrow_forward
- 11.51 Peroxyacetyl nitrate (PAN) has the chemical formula CtHjNOj and is an important lung irritant in photochemical smog. An experiment to determine the decomposition kinetics of PAN gave the data below. Determine the order of reaction and calculate the rate constant for the decomposition of PAN. Time, t (min) Partial Pressure of PAN (torr) 0.0 2.00 X 10~’ 10.0 1.61 X 10~} 20.0 1.30 X 10_J 30.0 1.04 X 10"’ 40.0 8.41 X 10-4 50.0 6.77 x 10-4 60.0 5.45 X 10-4arrow_forwardExperimental data are listed here for the reaction A 2 B. (a) Prepare a graph from these data; connect the points with a smooth line; and calculate the rate of change of [B] for each 10-second interval from 0.0 to 40.0 seconds. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result. (b) How is the rate of change of [A] related to the rate of change of [B] in each time interval? Calculate the rate of change of [A] for the time interval from 10.0 to 20.0 seconds.arrow_forwardExperimental data are listed here for the reaction A 2 B. (a) Prepare a graph from these data; connect the points with a smooth line; and calculate the rate of change of [B] for each 10-second interval from 0.0 to 40.0 seconds. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result. (b) How is the rate of change of [A] related to the rate of change of [B] in each time interval? Calculate the rate of change of [A] for the time interval from 10.0 to 20.0 seconds.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





