
Concept explainers
Interpretation:
The
Concept introduction:
There are primarily four types of acid-base titrations –
- Strong base vs strong acid
- Strong base vs weak acid
- Weak base vs strong acid
- Weak base vs weak acid
The term
Answer to Problem 11.AE
The
| S.No | Volume of
|
|
| 1. |
|
|
| 2. |
|
|
| 3. |
|
|
| 4. |
|
|
| 5. |
|
|
| 6. |
|
|
| 7. |
|
|
| 8. |
|
|
| 9. |
|
|
| 10. |
|
|
| 11. |
|
|
| 12. |
|
|
| 13. |
|
|
| 14. |
|
|
| 15. |
|
|
The graph of
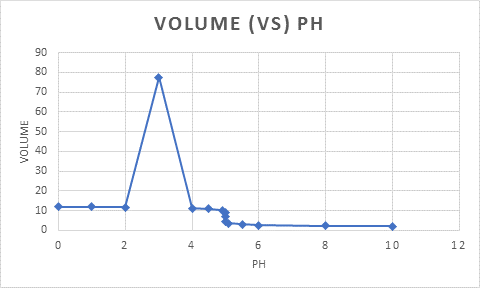
Figure 1
Explanation of Solution
Given that volume of acid,
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Let ‘x’ be the concentration of
Then,
After reaching the equivalence point, continual addition of acid increases the concentration of acid so that we need to calculate
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
When volume of acid added is
Substitute the values known to determine
The graph of

Figure 1
The
Want to see more full solutions like this?
Chapter 11 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





