
Interpretation:
Theoutline of a separation scheme for isolating caffeine from tea needs to be explained.
Concept Introduction:
In the isolation of caffeine from tea leaves, the main problem is that the caffeine is not the only natural substance present in the tea leaves, but there are other substances as well from which it needs to be separated. The main component is cellulose which is
Answer to Problem 1Q
Explanation of Solution
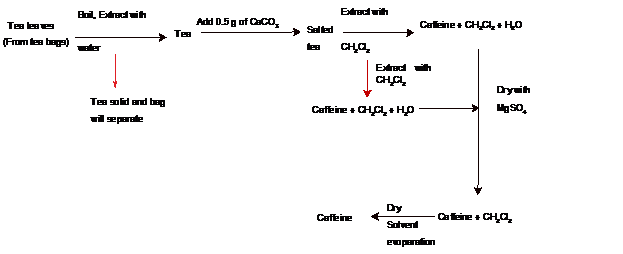
Caffeine is mainly extracted from tea leaves. There are certain steps of isolation which includes extraction and purification of caffeine from tea. The flow chart is as given below:

According to the above flow chart, tea leave from the tea bags are boiled and extracted with water. After that 0.5 g of
Thus, theseparation scheme for isolating caffeine from tea is represented as follows:

Want to see more full solutions like this?
Chapter 11 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Make a results and discussion in Laboratory Report Format of the following: 1. Separation of mixture using different techniques 1.1 Simple distillation: Water + acetone 1.2 Fractional Distillation: Methanol + ethanol 1.3 Chromatography: Separation of mixture of Red ink and blue ink 2. purification of impure samples by crystallization 2.1 Copper sulfate 2.2 Potash alum 2.3 Benzoic acidarrow_forwardAmino acids and fatty acids do not readily elute from gas chromatography columns even at temperatures above 200 degrees Celsius. What can be done to these biomolecules to allow gas chromatographic analysis?arrow_forward1. Why should the stopper always be removed from a separatory funnel whenever a liquid is being drained through the stopcock?2. The distribution coefficient, KD (ether/water), between ether and water for aspirin at room temperature is 3.5. What weight of aspirin would be extracted by a single extraction with 60 ml of water from a solution of 10 grams of aspirin in 100 ml of ether? Calculate the weight of aspirin which would be removed by three extractions with 20 ml portions of water.arrow_forward
- You are carrying out an experiment by analysing alcoholic drinks by gas chromatography. Why is Gas chromatography the best technique used for this analysis? Why is Carbowax a suitable stationary phase for this analysis? What gases are used and what is their purpose and source?arrow_forwardIs TLC chromatography and paper chromatography in a normal phase chromatography or reverse phase chromatography? Explain using its mobile and stationary phase.arrow_forwardWhat is the minimum distribution constant that permits removal of 99% of a solute from 50.0 mL of water with two 25.0-mL extractions with toluene? five 10.0-mL extractions with toluene?arrow_forward
- is 2% NaCl is more polar than the whatman filter paper?arrow_forwardWhat is the main drawback of using steam distillation in the isolation of volatile oils from its matrix?arrow_forwarda) What would you see if you ran a Thin Layer Chromatography (TLC) on a mixture of the three solids shown below using silica gel as the stationary phase and ethyl acetate/hexanes as the mobile phase? Whichsolid would have the highest Rf and which would have the lowest Rf? Explain b) Would changing the mobile phase (from part a) and increasing thepolarity by introducing small amount of methanol to the mobile phase change the order ofRf of the above 3 compounds? Explain c) Another type of chromatography is Reverse Phase Chromatography. In this type ofchromatography, the stationary phase is non-polar and the mobile phase is polar. If astudent performed Reverse Phase TLC on the mixture of 3 solids (from part a),what would be the order of Rf’s of the compounds? Which would have the lowest Rf andwhich would have the highest Rf?arrow_forward
- Correlate the structures of the standard food dyes to their relative position in the paper chromatogram. Refer to the structures shown below. What are the identities of the unknowns in paper chromatography of food dyes? How did you arrive at this conclusion?arrow_forwardBased on the resulting chromatograms after visualization with iodine vapor, which between the solvent systems WHITE and MIX resulted in a more effective separation of the pigments of the indigo extract? Explain your answer in terms of the solvent that did not effectively separate the components of the root extract.arrow_forwardExtraction of the aqueous salicylic acid solutions with 10% ethyl acetate/hexane (density ~ 0.7 g/ml) will give two layers in the separatory funnel. a. Will the aqueous layer be the upper layer or the lower layer? b. If dichloromethane (density ~ 1.3 g/ml) were substituted for the 10% ethyl acetate/hexane solution, which layer would be the aqueous layer?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

