
Equilibrium vapor pressures of benzene, C6H6, at various temperatures are given in the table.
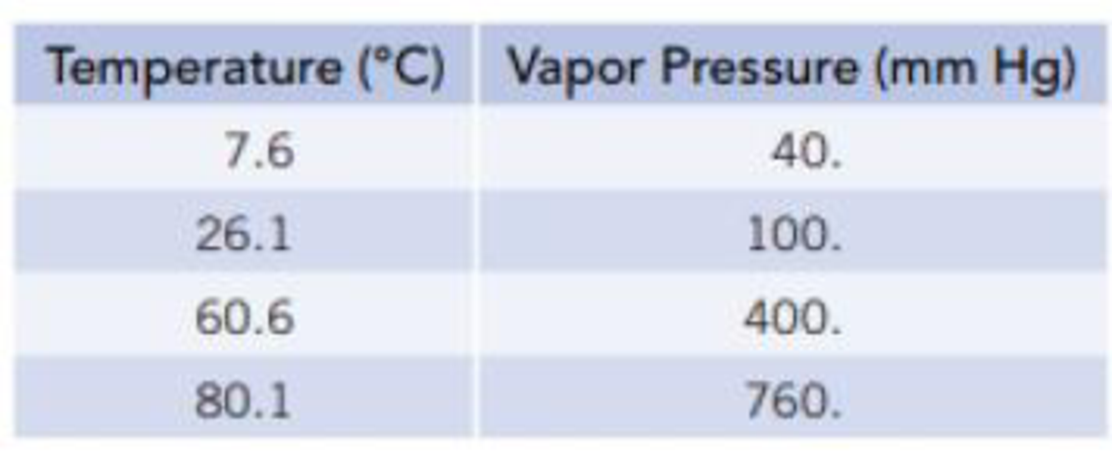
- (a) What is the normal boiling point of benzene?
- (b) Plot these data so that you have a graph resembling the one in Figure 11.12. At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg? At what temperature is the vapor pressure 650 mm Hg?
- (c) Calculate the molar enthalpy of vaporization for benzene using the Clausius–Clapeyron equation.
(a)
Interpretation:
The normal boiling point of benzene has to be determined.
Concept Introduction:
Boiling point: It is the temperature at which liquid converts to vapor. At boiling point the vapor pressure of liquid and the pressure of the surroundings are equal.
Normal boiling point: When the external pressure is
Answer to Problem 21PS
The normal boiling point of benzene is
Explanation of Solution
The normal boiling point of benzene is calculated
Given:
Normal boiling point is the temperature when the external pressure is
From the given data it is clear that the temperature at which the pressure is
Thus the normal boiling point of benzene is
(b)
Interpretation:
The temperature versus vapor pressure graph should be plotted. The temperatures at which the liquid has vapour pressures of
Concept Introduction:
Vapor pressure is nothing but the pressure of a vapor in contact with its liquid or solid form.
When a liquid and vapor are in equilibrium the pressure exerted by the vapor is called the equilibrium vapor pressure
Answer to Problem 21PS
The temperatures at which liquid have a vapour pressures of
Explanation of Solution
Given,
The temperatures at which liquid have a vapour pressures of
Using the given data we can plot the graph of
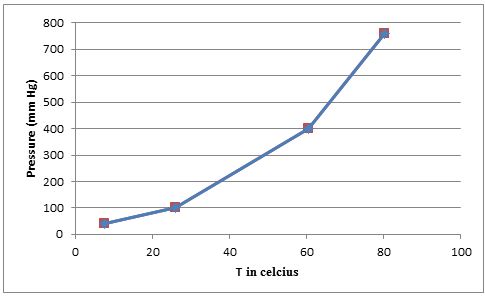
From the graph we can find the approximate temperatures at which the pressures are
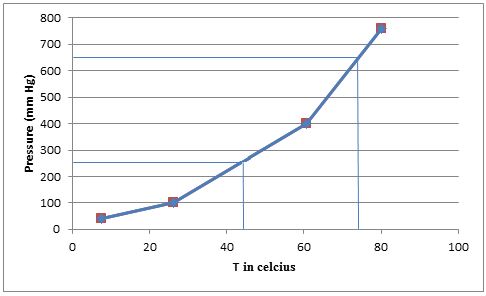
Therefore,
The temperature at which the pressure is
(c)
Interpretation:
The molar enthalpy of vaporization using Clausius-Clapeyron has to be determined
Concept Introduction:
Clausius-Clapeyron equation:
From this relationship we can calculate the molar enthalpy of vaporization by knowing the corresponding temperature and pressure values.
If we have pressures at two different temperatures, then enthalpy of vaporization can be calculated by
Answer to Problem 21PS
The molar enthalpy of vaporization of is
Explanation of Solution
The molar enthalpy of vaporization is calculated using the given data,
Given:
Clausius-Clapeyron equation is,
Substituting the values
The molar enthalpy of vaporization of is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry (3rd Edition)
Elementary Principles of Chemical Processes, Binder Ready Version
Chemistry: The Central Science (14th Edition)
General Chemistry: Atoms First
- The vapor pressure of ethanol, C2H5OH, at 50.0 C is 233 mmHg, and its normal boiling point at 1 atm is 78.3 C. Calculate the vapH of ethanol.arrow_forwardConsider the following data for the vapor pressure of diethyl ether, a widely used anesthetic in the early days of surgery. Follow the instructions in Question 13 to estimate the heat of vaporization of diethyl ether.arrow_forwardDiethyl ether (CH3CH2OCH2CH3) was one of the first chemicals used as an anesthetic. At 34.6C, diethyl ether has a vapor pressure of 760. torr, and at 17.9C, it has a vapor pressure of 400. torr. What is the H of vaporization for diethyl ether?arrow_forward
- Chloroform, CHCl3, has a normal boiling point of 61C. Its vapor pressure at 43C is 0.526 atm. What is the concentration (in g/L) of CHCl3 when it saturates the air at 27C?arrow_forwardDefine critical temperature and critical pressure. In terms of the kinetic molecular theory, why is it impossible for a substance to exist as a liquid above its critical temperature?arrow_forward8.87 Use the vapor pressure curves illustrated here to answer the questions that follow. (a) What is the vapor pressure of ethanol (C2H5OH) at 60°C? (b) Considering only carbon disulfide (CS2) and ethanol, which has the stranger intermolecular forces in the liquid state? (c) At what temperature does heptane (C7H16) have a vapor pressure of 500 mm Hg? (d) What are the approximate normal boiling pains of each of the three substances? (e) At a pressure of 400 mm Hg and a temperature of 70°C, is each substance a liquid, a gas, or a mixture of liquid and gas?arrow_forward
- The following data are the equilibrium vapor pressure of limonene, C10H16, at various temperatures. (Limonene is used as a scent in commercial products.) (a) Plot these data as ln P versus 1/T so that you have a graph resembling the one in Figure 11.13. (b) At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg? At what temperature is it 650 mm Hg? (c) What is the normal boiling point of limonene? (d) Calculate the molar enthalpy of vaporization for limonene using the Clausius-Clapeyron equation.arrow_forwardUse Figure 11.7 to estimate the boiling point of diethyl ether, (C2H5)2O, under an external pressure of 470 mmHg.arrow_forward5-106 The normal boiling point of hexane, C6H14, is 69°C, and that of pentane, C5H12, is 36°C. Predict which of these compounds has a higher vapor pressure at 20°C.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





