
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 23QAP
For a reaction involving the decomposition of Z at a certain temperature, the following data are obtained:
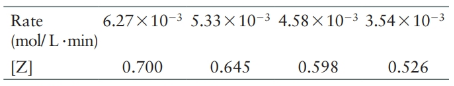
(a) What is the order of the reaction?
(b) Write the rate expression for the decomposition of Z.
(c) Calculate k for the decomposition at that temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Chemistry: Principles and Reactions
Ch. 11 - Express the rate of the reaction...Ch. 11 - Express the rate of the reaction...Ch. 11 - Consider the following hypothetical reaction: X( g...Ch. 11 - Consider the following hypothetical reaction:...Ch. 11 - Consider the combustion of ethane:...Ch. 11 - For the reaction 5Br(aq)+BrO3(aq)+6...Ch. 11 - Nitrosyl chloride (NOCI) decomposes to nitrogen...Ch. 11 - Ammonia is produced by the reaction between...Ch. 11 - Experimental data are listed for the following...Ch. 11 - Experimental data are listed for the hypothetical...
Ch. 11 - A reaction has two reactants X and Y. What is the...Ch. 11 - A reaction has two reactants Q and P. What is the...Ch. 11 - What will the units of the rate constants in...Ch. 11 - What will the units of the rate constants in...Ch. 11 - Consider the reaction ZproductsThe data below give...Ch. 11 - Consider the reaction YproductsThe graph below...Ch. 11 - Complete the following table for the reaction...Ch. 11 - Complete the following table for the reaction...Ch. 11 - The decomposition of nitrogen dioxide is a...Ch. 11 - The decomposition of ammonia on tungsten at 1100C...Ch. 11 - The reaction ICl(g)+12 H2(g)12 I2(g)+HCl(g)is...Ch. 11 - The hypothetical reaction X(g)+12Y(g)productsis...Ch. 11 - For a reaction involving the decomposition of Z at...Ch. 11 - For a reaction involving the decomposition of Y,...Ch. 11 - When boron trifluoride reacts with ammonia, the...Ch. 11 - When nitrogen dioxide reacts with carbon monoxide,...Ch. 11 - Hydrogen bromide is a highly reactive and...Ch. 11 - Diethylhydrazine reacts with iodine according to...Ch. 11 - The equation for the reaction between iodide and...Ch. 11 - Prob. 30QAPCh. 11 - In a solution at a constant H+ concentration,...Ch. 11 - Consider the reaction Â...Ch. 11 - Nitrosyl bromide decomposes to nitrogen oxide and...Ch. 11 - Prob. 34QAPCh. 11 - Azomethane decomposes into nitrogen and ethane at...Ch. 11 - The decomposition of sulfuryl chloride, SO2Cl2, to...Ch. 11 - The first-order rate constant for the...Ch. 11 - Consider the first-order decomposition of phosgene...Ch. 11 - The decomposition of azomethane, (CH3)2N2, to...Ch. 11 - The first-order rate constant for the...Ch. 11 - In the first-order decomposition of acetone at...Ch. 11 - The decomposition of sulfuryl chlorideSO2Cl2fur...Ch. 11 - Dinitrogen pentoxide gas decomposes to form...Ch. 11 - Sucrose (C12H22O11) hydrolyzes into glucose and...Ch. 11 - Iodine-131 is used to treat tumors in the thyroid....Ch. 11 - Cesium-131 is the latest tool of nuclear medicine....Ch. 11 - Prob. 47QAPCh. 11 - A sample of sodium-24 chloride contains 0.050 mg...Ch. 11 - The decomposition of A at 850C is a zero-order...Ch. 11 - The decomposition of R at 33C is a zero-order...Ch. 11 - For the zero-order decomposition of HI on a gold...Ch. 11 - For the zero-order decomposition of ammonia on...Ch. 11 - Ammonium cyanate, NH4NCO, in water rearranges to...Ch. 11 - Butadiene, C4H6, dimerizes according to the...Ch. 11 - The rate constant for the second-order reaction...Ch. 11 - The decomposition of nitrosyl chloride...Ch. 11 - An increase in temperature from 23C to 36C...Ch. 11 - If the activation energy of a reaction is 9.13 kJ,...Ch. 11 - The following data are obtained for the gas-phase...Ch. 11 - The following data are obtained for the...Ch. 11 - Consider the following hypothetical reaction:...Ch. 11 - For the reaction: Q+RY+ZH=128kJ Draw a...Ch. 11 - The uncoiling of deoxyribonucleic acid (DNA) is a...Ch. 11 - The precipitation of egg albumin in water at 100C...Ch. 11 - Prob. 65QAPCh. 11 - Prob. 66QAPCh. 11 - For the reaction 2N2O(g)2N2(g)+O2(g) the rate...Ch. 11 - For the decomposition of a peroxide, the...Ch. 11 - Consider a 5.000 M solution of the hypothetical...Ch. 11 - The decomposition of N2O5 to NO2 and NO3 is a...Ch. 11 - For a certain reaction, Ea is 135 kJ and H=45 kJ....Ch. 11 - Consider a reaction in which E a=129 kJ and H=29...Ch. 11 - A catalyst lowers the activation energy of a...Ch. 11 - A reaction has an activation energy of 363 kJ at...Ch. 11 - Write the rate expression for each of the...Ch. 11 - Write the rate expression for each of the...Ch. 11 - For the reaction between hydrogen and iodine,...Ch. 11 - For the reaction 2H2(g)+2NO(g)N2(g)+2H2O(g) the...Ch. 11 - At low temperatures, the rate law for the reaction...Ch. 11 - Two mechanisms are proposed for the reaction...Ch. 11 - The hypothetical reaction QR+Xproductswas...Ch. 11 - When a base is added to an aqueous solution of...Ch. 11 - The decomposition of sulfuryl chloride, SO2Cl2, to...Ch. 11 - How much faster would a reaction proceed at 46C...Ch. 11 - Prob. 85QAPCh. 11 - Prob. 86QAPCh. 11 - A drug decomposes in the blood by a first-order...Ch. 11 - Prob. 88QAPCh. 11 - Prob. 89QAPCh. 11 - Prob. 90QAPCh. 11 - Consider the decomposition of A represented by...Ch. 11 - Consider the decomposition reaction 2X2Y+ZThe...Ch. 11 - Consider the following activation energy diagram....Ch. 11 - Three first-order reactions have the following...Ch. 11 - Consider the first-order decomposition reaction...Ch. 11 - Consider the following energy diagram (not to...Ch. 11 - Prob. 97QAPCh. 11 - Prob. 98QAPCh. 11 - The gas-phase reaction between hydrogen and iodine...Ch. 11 - Consider the coagulation of a protein at 100C. The...Ch. 11 - Prob. 101QAPCh. 11 - Prob. 102QAPCh. 11 - Prob. 103QAPCh. 11 - In a first-order reaction, suppose that a quantity...Ch. 11 - Consider the hypothetical first-order reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a reaction involving the decomposition of a hypothetical substance Y, these data are obtained: Determine the order of the reaction. Write the rate law for the decomposition of Y. Calculate k for the experiment above.arrow_forwardSucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forwardWhen boron trifluoride reacts with ammonia, the following reaction occurs: BF3(g)+NH3(g)BF3NH3(g)The following data are obtained at a particular temperature: (a) What is the order of the reaction with respect to BF3, NH3, and overall? (b) Write the rate expression for the reaction. (c) Calculate k for the reaction. (d) When [ BF3 ]=0.533M and NH3=0.300M, what is the rate of the reaction at the temperature of the experiment?arrow_forward
- The half-life of tritium, 3H, is 12.26 years. Tritium is the radioactive isotope of hydrogen. (a) What is the rate constant for the radioactive decay of tritium, in y1 and s1? (b) What percentage of the original tritium is left after 61.3 years?arrow_forwardThe hydrolysis of the sugar sucrose to the sugars glucose and fructose, C12H22O11+H2OC6H12O6+C6H12O6 follows a first-order rate equation for the disappearance of sucrose: Rate =k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.) (a) In neutral solution, k=2.11011s1 at 27 C and 8.51011s1 at 37 C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature). (b) When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65107M . How long will it take the solution to reach equilibrium at 27 C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible. (c) Why does assuming that the reaction is irreversible simplify the calculation in pan (b)?arrow_forwardWhen nitrogen dioxide reacts with carbon monoxide, the following reaction occurs. Â NO2(g)+CO(g)NO(g)+CO2(g)The following data are obtained at a certain temperature: (a) What is the order of the reaction with respect to NO2, CO, and overall? (b) Write the rate expression of the reaction. (c) Calculate k for the reaction. (d) When [ NO2 ]=0.421Mand [ CO ]=0.816M, what is the rate of the reaction at the temperature of the experiments?arrow_forward
- At 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forwardRate data were obtained at 25 C for the following reaction. What is the rate-law expression for this reaction? A+2BC+2Darrow_forwardIf the activation energy of a reaction is 9.13 kJ, then what is the percent increase in the rate constant when the temperature is increased from 27C to 69C?arrow_forward
- Express the rate of the reaction 2N2O(g)2N2(g)+O2(g) in terms of (b) [ N2O ] (a) [ O2 ]arrow_forwardUnder certain conditions the decomposition of ammonia on a metal surface gives the following data: [NH3] (M) 1.0103 2.0103 3.0103 Rate (moI/L/h1) 1.5106 1.5106 1.5106 Determine the rate equation, the rate constant, and the overall order for this reaction.arrow_forwardThe initial rate ( [NO]/ t] of the reaction of nitrogen monoxide and oxygen NO(g) + 2O2(g) NO2(g) was measured for various initial concentrations of NO and O2 at 25 C. Determine the rate equation from these data. What is the value of the rate constant, k, and what are its units?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY