
Concept explainers
(a)
Interpretation:
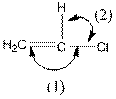
The indicated bond angle in the following compound should be predicted:
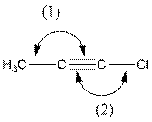
Concept Introduction:
The following table should be used while determining the bond around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear |
|
| 3 | 3 | 0 | Trigonal planar |
|
| 4 | 4 | 0 | Tetrahedral |
|
| 4 | 3 | 1 | Trigonal pyramidal |
|
| 4 | 2 | 2 | Bent |
|
Answer to Problem 27P
Angle (1) and angle (2) both measures
Explanation of Solution
The given structure is as follows:
In the compound, triple bonded carbon is bonded to two other groups. If two groups surround an atom and no lone pair electron are present, the bond angle is
(b)
Interpretation:
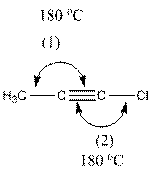
The indicated bond angle in the following compound should be predicted:
Concept Introduction:
The following table should be used while determining the bond angle around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear |
|
| 3 | 3 | 0 | Trigonal planar |
|
| 4 | 4 | 0 | Tetrahedral |
|
| 4 | 3 | 1 | Trigonal pyramidal |
|
| 4 | 2 | 2 | Bent |
|
Answer to Problem 27P
Angle (1) and (2) both measures
Explanation of Solution
The compound is as follows:
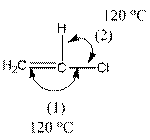
In the compound, three groups (hydrogen, carbon and chlorine) surround the double bonded carbon atom. And if three groups present surrounding an atom, the bond angle will be
(c)
Interpretation:
The indicated bond angle in the following compound should be predicted:
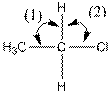
Concept Introduction:
The following table should be used while determining the bond angle around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear |
|
| 3 | 3 | 0 | Trigonal planar |
|
| 4 | 4 | 0 | Tetrahedral |
|
| 4 | 3 | 1 | Trigonal pyramidal |
|
| 4 | 2 | 2 | Bent |
|
Answer to Problem 27P
Angle (1) and angle (2) both measures
Explanation of Solution
Given compound is as follows:
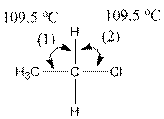
The carbon atom in the compound is surrounded by four groups (two hydrogen, one carbon and one chlorine). So, the angle between the bonds is
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- All of the following compounds exhibit geometric isomerism except: CH2=CH-CH3 CFCl=CHBr CH3ClC=CBrCH3 CHBr=CHClarrow_forwardPredict the geometry around each indicated atom. Give the orbital hybridization around each indicated atom. Predict the polarity around each indicated atom.arrow_forwardList these compounds in order of increasing carbon–carbon bondstrength and in order of decreasing carbon–carbon bond length:HCCH, H2CCH2, H3CCH3.arrow_forward
- Order these compounds in order of increasing carbon–carbon bond strength and in order of decreasing carbon–carbon bond length: HCCH, H2CCH2, H3CCH3.arrow_forwardThere are at least three different molecules with the formula C3H8O. Draw a Lewis structure for each possible constitutional isomer. Be careful not to duplicate any structures.arrow_forwardSelect the choice that best describes the relationship of the pair of compounds.arrow_forward
- How many hydrogen atoms are required to complete the structure of the compound below?arrow_forwardDraw at least four compounds of formula C4H6NOCl.arrow_forwardFor which compounds can a second resonance structure be drawn?Draw an additional resonance structure and the hybrid for eachresonance-stabilized compound.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
