
Concept explainers
Convert each shorthand structure to a complete structure with all atoms and lone pairs drawn in.
-
a.
b.
c.
d. 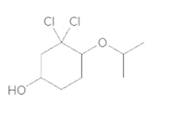
(a)
Interpretation:
To convert the following shorthand structure to complete structure with all atoms and lone pairs drawn.
Concept Introduction:
Complete structure of a compound is the one in which all the bonds, atoms and lone pairs are shown. Example of complete structure is given below.
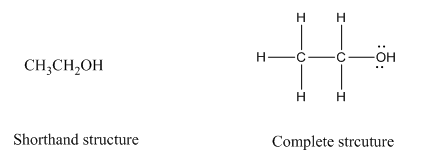
In shorthand structure, bond between the atoms and lone pairs of atoms are not shown. But, in complete structure, all the bonds between atoms and lone pairs are shown.
Answer to Problem 37P
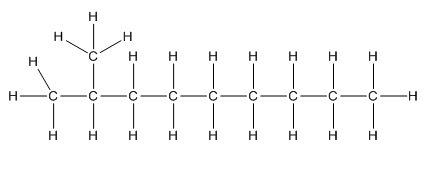
Complete structure of the compound is,
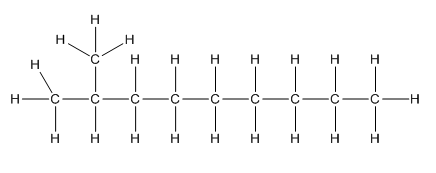
Explanation of Solution
Given compound is as follows:
To −CH group two methyl
(b)
Interpretation:
To convert the following shorthand structure to complete structure with all atoms and lone pairs drawn.
Concept Introduction:
Complete structure of a compound is the one in which all the bonds, atoms and lone pairs are shown. Example of complete structure is given below.
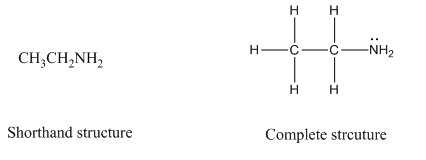
In shorthand structure, bond between the atoms and lone pairs of atoms are not shown. But, in complete structure, all the bonds between atoms and lone pairs are shown.
Answer to Problem 37P
Complete structure of the compound is as follows:
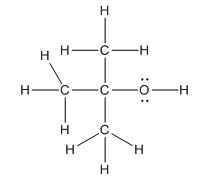
Explanation of Solution
In the compound
Hence, complete structure of the compound is as follows:
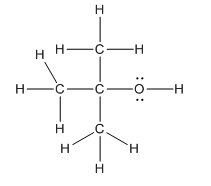
(c)
Interpretation:
To convert the following shorthand structure to complete structure with all atoms and lone pairs drawn.
Concept Introduction:
Complete structure of a compound is the one in which all the bonds, atoms and lone pairs are shown. Example of complete structure is given below.
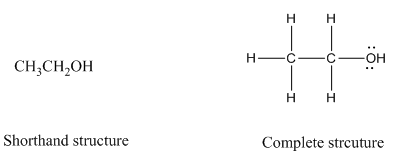
In shorthand structure, bond between the atoms and lone pairs of atoms are not shown. But, in complete structure, all the bonds between atoms and lone pairs are shown.
Answer to Problem 37P
Complete structure of the compound is as follows:
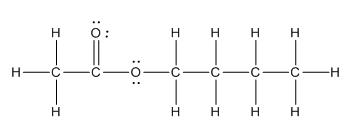
Explanation of Solution
Given compound is as follows:
In the complete structure, all the bonds between atoms and lone pairs are to be shown. Two oxygen atoms present. Both the oxygen atoms have two lone pairs (as the total number of valence electron of O is 6 and out of these 2 are involved in bond formation) on it. Hence, complete structure of the compound is as follows:
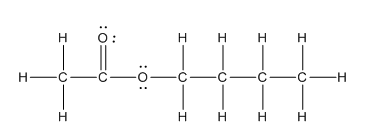
(d)
Interpretation:
To convert the following shorthand structure to complete structure with all atoms and lone pairs drawn.
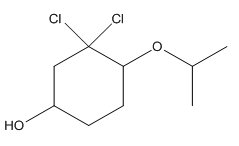
Concept Introduction:
Complete structure of a compound is the one in which all the bonds, atoms and lone pairs are shown. Example of complete structure is given below.
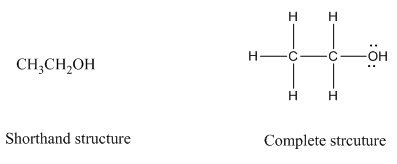
In shorthand structure, bond between the atoms and lone pairs of atoms are not shown. But, in complete structure, all the bonds between atoms and lone pairs are shown.
Answer to Problem 37P
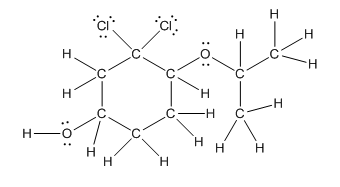
Complete structure is as follows:
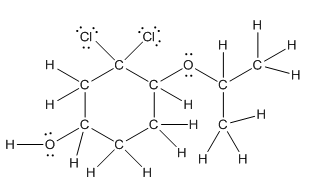
Explanation of Solution
Given compound is as follows:
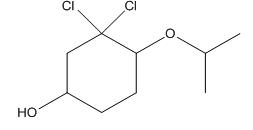
In complete structure, all the carbon atoms, hydrogen atoms and all the lone pairs should be shown. In the compound two chlorine atoms (as the total number of valence electron of Cl is 7 and out of these 1 is involved in bond formation), one oxygen atom and one −OH group(as the total number of valence electron of O is 6 and out of these 2 are involved in bond formation) present which has lone pairs. So, complete structure is as follows:
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- How would I convert each condensed formula to a lewis structure 1a. (CH3)2CHOCH2CH2CH2OH 1b. CH3(CH2)2CO2C(CH3)3arrow_forwardWhat is the total number of hydrogens in 2,3-dimethylhexane? a. 10 b.12 c.16 d.18arrow_forwardConvert each condensed formula to a Lewis structure. a.CH3(CH2)4CH(CH3)2 b. (CH3)3CCH(OH)CH2CH3 c. (CH3)2CHCHO d.(HOCH2)2CH(CH2)3C(CH3)2CH2CH3arrow_forward
- help with identifying the functional groups A-Harrow_forwardConvert each skeletal structure to a complete structure with all C's, H's, and lone pairs drawn in.arrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b.acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forward
- Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardConvert each condensed formula to a Lewis structure.1.) (CH3)2CHOCH2CH2CH2OH2.) CH3(CH2)2CO2C(CH3)3arrow_forwardHow many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





