
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 40IL
A “hand boiler” can be purchased in toy stores or at science supply companies. If you cup your hand around the bottom bulb, the volatile liquid in the boiler boils, and the liquid moves to the upper chamber.
- (a) Using your knowledge of kinetic molecular theory and intermolecular forces, explain how the hand boiler works.

- (b) Which of the following liquids would be best to use in the hand boiler? Explain.
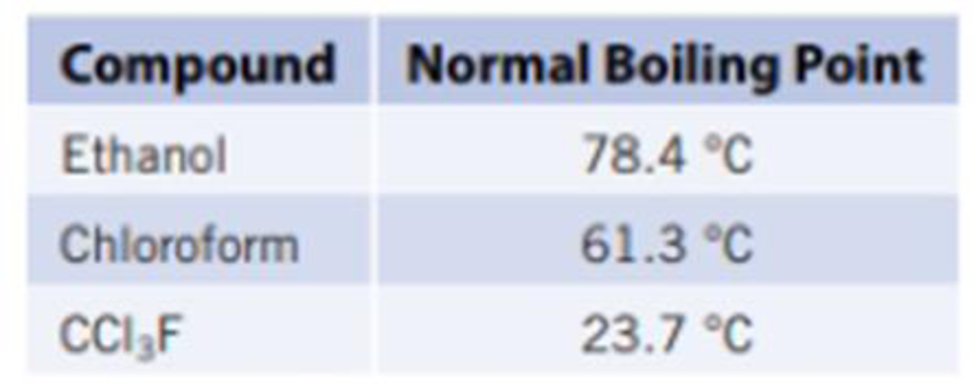
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 11 Solutions
Chemistry & Chemical Reactivity
Ch. 11.2 - Which should have the more negative hydration...Ch. 11.3 - Using structural formulas, describe the hydrogen...Ch. 11.4 - Prob. 11.3CYUCh. 11.5 - Prob. 11.4CYUCh. 11.6 - The molar enthalpy of vaporization of methanol,...Ch. 11.6 - Prob. 11.6CYUCh. 11.6 - Prob. 1.1ACPCh. 11.6 - Prob. 1.2ACPCh. 11.6 - Prob. 2.1ACPCh. 11.6 - Prob. 2.2ACP
Ch. 11 - Prob. 1PSCh. 11 - Intermolecular forces: What type of forces must be...Ch. 11 - Prob. 3PSCh. 11 - Prob. 4PSCh. 11 - Considering intermolecular forces in the pure...Ch. 11 - Considering intermolecular forces in the pure...Ch. 11 - Prob. 7PSCh. 11 - Which of the following compounds would be expected...Ch. 11 - Prob. 9PSCh. 11 - When salts of Mg2+, Na+, and Cs+ are placed in...Ch. 11 - Prob. 11PSCh. 11 - The enthalpy of vaporization of liquid mercury is...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Prob. 15PSCh. 11 - Refer to Figure 11.12 to answer these questions:...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Place the following four compounds in order of...Ch. 11 - Prob. 19PSCh. 11 - You are comparing three different substances, A,...Ch. 11 - Equilibrium vapor pressures of benzene, C6H6, at...Ch. 11 - Prob. 22PSCh. 11 - Can carbon monoxide (Tc = 132.9 K; Pc = 34.5 atm...Ch. 11 - Methane (CH4) cannot be liquefied at room...Ch. 11 - What is surface tension? Give an example...Ch. 11 - What factors affect the viscosity of a substance?...Ch. 11 - If a piece of filter paper (an absorbent paper...Ch. 11 - When water is placed in a buret it forms a concave...Ch. 11 - Prob. 29GQCh. 11 - What types of intermolecular forces are important...Ch. 11 - Which of the following salts, Li2SO4 or Cs2SO4, is...Ch. 11 - Prob. 32GQCh. 11 - Prob. 33GQCh. 11 - Prob. 34GQCh. 11 - Rank the following compounds in order of...Ch. 11 - Prob. 36GQCh. 11 - Prob. 37GQCh. 11 - The following data are the equilibrium vapor...Ch. 11 - Prob. 39ILCh. 11 - A hand boiler can be purchased in toy stores or at...Ch. 11 - Prob. 41ILCh. 11 - Prob. 42ILCh. 11 - Acetone, CH3COCH3, is a common laboratory solvent....Ch. 11 - Cooking oil floats on top of water. From this...Ch. 11 - Liquid ethylene glycol, HOCH2CH2OH, is one of the...Ch. 11 - Liquid methanol, CH3OH, is placed in a glass tube....Ch. 11 - Account for these facts: (a) Although ethanol...Ch. 11 - Prob. 48SCQCh. 11 - Prob. 49SCQCh. 11 - Prob. 50SCQCh. 11 - Prob. 51SCQCh. 11 - Prob. 52SCQCh. 11 - A fluorocarbon, CF4, has a critical temperature of...Ch. 11 - Prob. 55SCQCh. 11 - List four properties of liquids that are directly...Ch. 11 - List the following ions in order of hydration...Ch. 11 - Prob. 59SCQCh. 11 - An 8.82-g sample of Br2 is placed in an evacuated...Ch. 11 - Polarizability is defined as the extent to which...Ch. 11 - Prob. 62SCQCh. 11 - A pressure cooker (a kitchen appliance) is a pot...Ch. 11 - Vapor pressures of NH3() at several temperatures...Ch. 11 - Prob. 65SCQCh. 11 - Prob. 66SCQCh. 11 - Prob. 67SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Define the following and give an example of each: (a) dispersion force (b) dipole-dipole attraction (c) hydrogen bondarrow_forwardOn the basis of intermolecular attractions, explain the differences in the boiling points of n butane (1 C) and chloroethane (12 C), which have similar molar masses.arrow_forwardWhat are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.arrow_forward
- 5-106 The normal boiling point of hexane, C6H14, is 69°C, and that of pentane, C5H12, is 36°C. Predict which of these compounds has a higher vapor pressure at 20°C.arrow_forwardIn terms of the kinetic molecular theory, in what ways are liquids similar to solids? In what ways are liquids different from solids?arrow_forwardClassify each of the following statements as true or false. a Intermolecular attractions are stronger in liquids than in gases. b Substances with weak intermolecular attractions generally have low vapor pressures. c Liquids with high molar heats of vaporization usually are more viscous than liquids with low molar heats of vaporization. d A substance with a relatively high surface tension usually has a very low boiling point. e All other things being equal, hydrogen bonds are weaker than induced dipole or dipole forces. f Induced dipole forces become very strong between large molecules. g Other things being equal, nonpolar molecules have stronger intermolecular attractions than polar molecules. h The essential feature of a dynamic equilibrium is that the rates of opposing changes are equal. i Equilibrium vapor pressure depends on the concentration of a vapor above its own liquid. j The heat of vaporization is equal to the heat of fusion, but with opposite sign. k The boiling point of a liquid is a fixed property of the liquid. l If you break shatter an amorphous solid, it will break in straight lines, but if you break a crystalline solid, it will break in curved lines. m Ionic crystals are seldom soluble in water. n Molecular crystals are nearly always soluble in water. o The numerical value of heat of vaporization is always larger than the numerical value of heat of condensation. p The units of heat of fusion are kJ/gC. q The temperature of water drops while it is freezing. r Specific heat is conerned with a change in temperature.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY