
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 11, Problem 43SCQ
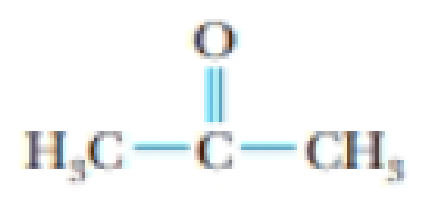
Acetone, CH3COCH3, is a common laboratory solvent. It is usually contaminated with water, however. Why does acetone absorb water so readily? Draw molecular structures showing how water and acetone can interact. What intermolecular force(s) is(are) involved in the interaction?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
6. If the molality of the following compounds is the same, which compound will have the highest boiling point in water is......
(A) CaCl2
(B) NaBr
(C) CuSO4
(D) CH3OH
(E) KNO3
1.) Are there any intermolecular forces (IMF’s) between water molecules and cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of DHMIXING be for cyclohexane dissolving in water?
a) Are there any IMF’s between water molecules and other water molecules? What kind(s)?
Given this, what would the magnitude and sign of DHSOLVENT be for cyclohexane dissolving in water?
c) Are there any IMF’s between cyclohexane molecules and other cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of DHSOLUTE be for cyclohexane dissolving in water?
d) Why do you think cyclohexane does not dissolve in water? Analyze this the way it was done in class, thinking about the three contributions to DHSOLUTION. What do you think the magnitude and sign of DHSOLUTION would be for dissolving cyclohexane in water? (Explain.) Would you expect these substances to dissolve in each other? Why?
What is the respiration rate for a breath every 2.5 seconds?
What solids are made up of large numbers of independent, constituent units, some of which attract and others repel.
Chapter 11 Solutions
Chemistry & Chemical Reactivity
Ch. 11.2 - Which should have the more negative hydration...Ch. 11.3 - Using structural formulas, describe the hydrogen...Ch. 11.4 - Prob. 11.3CYUCh. 11.5 - Prob. 11.4CYUCh. 11.6 - The molar enthalpy of vaporization of methanol,...Ch. 11.6 - Prob. 11.6CYUCh. 11.6 - Prob. 1.1ACPCh. 11.6 - Prob. 1.2ACPCh. 11.6 - Prob. 2.1ACPCh. 11.6 - Prob. 2.2ACP
Ch. 11 - Prob. 1PSCh. 11 - Intermolecular forces: What type of forces must be...Ch. 11 - Prob. 3PSCh. 11 - Prob. 4PSCh. 11 - Considering intermolecular forces in the pure...Ch. 11 - Considering intermolecular forces in the pure...Ch. 11 - Prob. 7PSCh. 11 - Which of the following compounds would be expected...Ch. 11 - Prob. 9PSCh. 11 - When salts of Mg2+, Na+, and Cs+ are placed in...Ch. 11 - Prob. 11PSCh. 11 - The enthalpy of vaporization of liquid mercury is...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Prob. 15PSCh. 11 - Refer to Figure 11.12 to answer these questions:...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Place the following four compounds in order of...Ch. 11 - Prob. 19PSCh. 11 - You are comparing three different substances, A,...Ch. 11 - Equilibrium vapor pressures of benzene, C6H6, at...Ch. 11 - Prob. 22PSCh. 11 - Can carbon monoxide (Tc = 132.9 K; Pc = 34.5 atm...Ch. 11 - Methane (CH4) cannot be liquefied at room...Ch. 11 - What is surface tension? Give an example...Ch. 11 - What factors affect the viscosity of a substance?...Ch. 11 - If a piece of filter paper (an absorbent paper...Ch. 11 - When water is placed in a buret it forms a concave...Ch. 11 - Prob. 29GQCh. 11 - What types of intermolecular forces are important...Ch. 11 - Which of the following salts, Li2SO4 or Cs2SO4, is...Ch. 11 - Prob. 32GQCh. 11 - Prob. 33GQCh. 11 - Prob. 34GQCh. 11 - Rank the following compounds in order of...Ch. 11 - Prob. 36GQCh. 11 - Prob. 37GQCh. 11 - The following data are the equilibrium vapor...Ch. 11 - Prob. 39ILCh. 11 - A hand boiler can be purchased in toy stores or at...Ch. 11 - Prob. 41ILCh. 11 - Prob. 42ILCh. 11 - Acetone, CH3COCH3, is a common laboratory solvent....Ch. 11 - Cooking oil floats on top of water. From this...Ch. 11 - Liquid ethylene glycol, HOCH2CH2OH, is one of the...Ch. 11 - Liquid methanol, CH3OH, is placed in a glass tube....Ch. 11 - Account for these facts: (a) Although ethanol...Ch. 11 - Prob. 48SCQCh. 11 - Prob. 49SCQCh. 11 - Prob. 50SCQCh. 11 - Prob. 51SCQCh. 11 - Prob. 52SCQCh. 11 - A fluorocarbon, CF4, has a critical temperature of...Ch. 11 - Prob. 55SCQCh. 11 - List four properties of liquids that are directly...Ch. 11 - List the following ions in order of hydration...Ch. 11 - Prob. 59SCQCh. 11 - An 8.82-g sample of Br2 is placed in an evacuated...Ch. 11 - Polarizability is defined as the extent to which...Ch. 11 - Prob. 62SCQCh. 11 - A pressure cooker (a kitchen appliance) is a pot...Ch. 11 - Vapor pressures of NH3() at several temperatures...Ch. 11 - Prob. 65SCQCh. 11 - Prob. 66SCQCh. 11 - Prob. 67SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 1.40-g sample of polyethylene, a common plastic, is dissolved in enough organic solvent to give 100.0 mL of solution. What is the average molar mass of the polymer if the measured osmotic pressure of the solution is 1.86 mm Hg at 25 C?arrow_forwardA solution of benzoic acid in benzene has a freezing point of 3.1 C and a boiling point of 82.6 C. (The freezing point of pure benzene is 5.50 C, and its boiling point is 80.1 C) The structure of benzoic acid is Benzoic acid, C6H5CO2H What can you conclude about the state of the benzoic acid molecules at the two different temperatures? Recall the discussion of hydrogen bonding in Section 11.3.arrow_forwardWhen sails of Mg2+, Ca2+, and Be2+ are placed in water, the positive ion is hydrated (as is the negative ion). Which of these three cations is most strongly hydrated? Which one is least strongly hydrated?arrow_forward
- These modify the interfacial properties of substances. * a. Aerosols b. Suspensions c, Surfactants d. Nanotechnologyarrow_forwardConsider the two forms of a non-biologically important amino acid shown below. What is the biggest difference between the two in terms of the structures and therefore the intermolecular forces they experience in aqueous solutions?arrow_forwardIf 11.2 g of sugar (MM= 342 g/mol) is dissolved in 500 g water at 30 degrees celsius, a. how many grams of sugar must be added to 1000 g water to make it boil at 102.0 degrees celsius? b. how many grams of sugar must be added to 1000 g water to make it freeze at -2.000 degrees celsius? c. what is the molar mass of a 500.0 g solute which then dissolved in 500.0 g water results in a solution that boils at 105.5 degrees celsius? kindly answer a-carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY