
Concept explainers
Methyl salicylate is responsible for the characteristic odor of the oil Wintergreen.
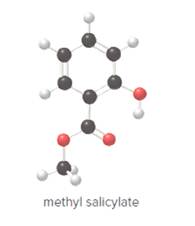
- Give the molecular formula for methyl salicylate.
- Draw in all lone pairs on heteroatoms using a skeletal structure.
- How many trigonal planar carbons does methyl salicylate contain?
- Predict the water solubility of methyl salicylate.
- Label all polar bonds.
(a)
Interpretation:
To determine the molecular formula of methyl salicylate from the ball and stick model.
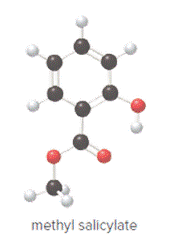
Concept Introduction:
In ball and stick model, black color ball represents carbon atom, white color ball represents hydrogen atom, red color ball represents oxygen atom and blue color ball represents nitrogen atom. To determine the molecular formula, convert the ball and stick model to complete structure.
Answer to Problem 82P
Molecular formula of methyl salicylate is
Explanation of Solution
Ball and stick model of methyl salicylate is as follows:
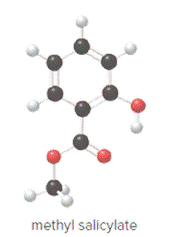
Convert ball and stick model to normal structure. Replace black balls by carbon atoms, red balls by oxygen atoms and white balls by hydrogen atom. But all the bonds between atoms are same.
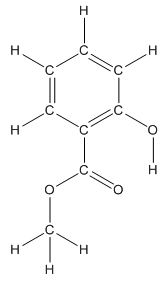
In methyl salicylate, eight carbon atoms, eight hydrogen atoms and three oxygen atoms present. Hence, molecular formula of methyl salicylate is
(b)
Interpretation:
To draw all lone pairs on heteroatoms of methyl salicylate using a skeletal structure.
Concept Introduction:
Heteroatoms are those atoms in an organic compound other than carbon and hydrogen atom like oxygen, nitrogen, etc. Lone pairs are pair of electrons available on an atom after bond formation.
Answer to Problem 82P
The structure having all lone pairs on heteroatoms is represented as follows:
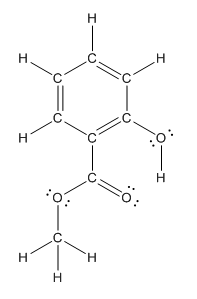
Explanation of Solution
Structure of methyl salicylate is as follows:
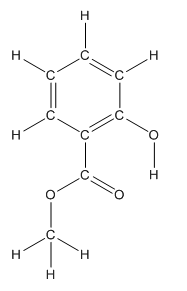
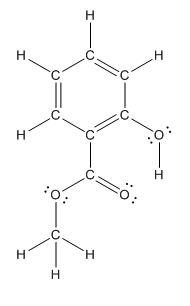
The heteroatoms present in benzocaine are oxygen atoms. Oxygen has six valence electrons. In double bonded oxygen atom, two electrons are used for making double bond. Remaining four electrons present as two lone pairs. Both the single bonded oxygen atom has two bonds. So, remaining four electrons present as two lone pairs in both the oxygen atom. So, the structure having all lone pairs on heteroatoms is represented as follows:
(c)
Interpretation:
To determine the number of carbon atoms having trigonal planar shape in methyl salicylate.
Concept Introduction:
The following table should be used while determining shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Answer to Problem 82P
Seven carbon atoms in methyl salicylate have trigonal planar structure.
Explanation of Solution
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Structure ofmethyl salicylate is as follows:
To find the number of carbon atoms having trigonal planar shape in methyl salicylate, observe the carbon atoms and find which carbon atom has three groups surround it.
All the carbon atoms in the benzene ring have three atoms around them also the carbon bonded to the benzene ring has three groups surround it. So, seven carbon atoms in methyl salicylatehave trigonal planar structure represented as follows:
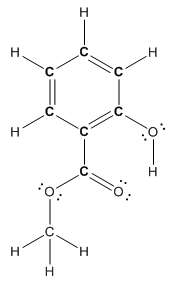
The bold carbon atoms in the above structure have trigonal planar structure.
(d)
Interpretation:
To predict the solubility of methyl salicyate in water.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 82P
Methyl salicylate is soluble in water.
Explanation of Solution
Structure of methyl salicylate is as follows:
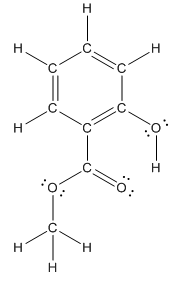
In methyl salicylate, three heteroatoms present that is, three oxygen atoms. These heteroatoms can form hydrogen bond with water. The presence of heteroatoms make the compound polar and a polar compound will dissolve in polar compound. So, methyl salicylate is soluble in water.
(e)
Interpretation:
To label all the polar bonds in methyl salicylate.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 82P
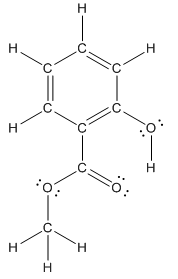
The structure of methyl salicylate with all polar bonds labeled is as follows:
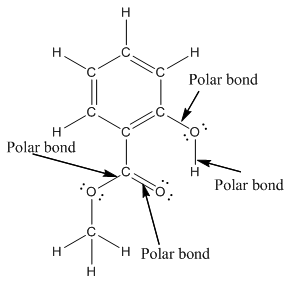
Explanation of Solution
Structure of methyl salicylate is as follows:
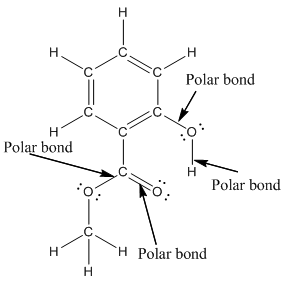
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc. In methyl salicylate, four polar bonds present that is, three polar bond between carbon and oxygen (oxygen is more electronegative than carbon) and one polar bond between oxygen and hydrogen (oxygen is more electronegative than hydrogen).
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- How many electron pairs are shared when a triple bond exists between two carbon atoms? What must he the geometric arrangement around the carbon atoms in a triple bond? Draw the Lewis structure of a simple molecule that contains a triple bond.arrow_forwardDraw the skeletal structure of 2,2-dimethylheptane from the condensed formula (shown below). CH3C(CH3)2CH2CH2CH2CH2CH3arrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b.acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forward
- Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c.dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d.acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardDraw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH2)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH2CHCN, starting material used to manufacture synthetic Orlon fibers c. dihydroxyacetone, (HOCH2)2CO, an ingredient in sunless tanning products d. acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forwardwhat is the condensed molecular formula of 3 ethyl 2 methyl nonanearrow_forward
- Answer this question:How to draw the line bond formula or lewis structure of a methyl ketone with the chemical formula C6H5C3H5O? (also taking into consideration the solubility test and chemical test results provided below) Based on the results of the solubility tests the compound is insoluble in water, 10% NaOH and 10% HCl but soluble in concentrated H2SO4. The functional group/class is identified to be Methyl Ketone, based on the results of the chemical tests on Table 2. CHEMICAL TEST OBSERVATIONS +(compound tested positive for the chemical reaction)/ otherwise (-) Molisch test turbid colorless solution - 2,4-DNP test formation of orange-yellow precipitates + Tollen’s test turbid colorless solution - Ninhydrin test clear pale-yellow solution - iodoform test clear pale-yellow solution +arrow_forwardIdentify the functional groups in each molecule. Classify each alcohol, alkyl halide, amide, and amine as 1°, 2°, or 3° ?arrow_forwardOrganic compounds may have characteristic odors as well as other characteristic physical properties. For example, the distinct odor of the seashore at low tide results in part from the presence of dimethyl sulfide (CH3SCH3), a molecule with a similar structure to dimethyl ether (CH3OCH3). Ethanethiol (CH3CH2SH), also called mercaptan, is an isomer of dimethyl sulfide with a much less pleasant odor.The table lists four related compounds and their enthalpies of vaporization (ΔH°vap) in kJ/mol. Compound ΔH°vap (kJ/mol) CH3OCH3 23 CH3SCH3 28 CH3CH2SH 27.5 CH3CH2OH 42 Rank the following compounds in order of increasing strength of their intermolecular forces, given the ΔH°vap listed for each. Place the compound with the strongest intermolecular forces (IMFs) at the top of the list. (Strongest to weaknest). Why is ΔHºvap for CH3SCH3 greater than ΔHºvap for CH3OCH3? A. CH3OCH3 is more polar. B. CH3SCH3 has stronger dipole–dipole attractions. C. CH3OCH3 can form…arrow_forward
- Indicate the number of bonds and lone pairs of electrons on each of the following atoms: (a) a neutral nitrogen (b) a negatively charge nitrogen (c) a negatively charged carbon (d) a positively charged oxygenarrow_forwardGive an example (condensed and expanded structures, and line-angle formulas) of each of the following classes of compounds with at least 6 carbon atoms. Name each compound. b. Alcoholarrow_forwardConvert each condensed formula to a Lewis structure.1.) (CH3)2CHOCH2CH2CH2OH2.) CH3(CH2)2CO2C(CH3)3arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
