
Dicarbonyl compounds such as quinones are reactive dienophiles.
(a)

(b)
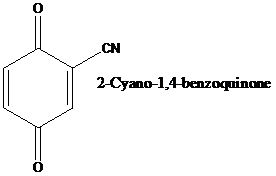
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Organic Chemistry - Standalone book
- 5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardThe bicyclic alkene P can be prepared by thermal electrocyclic ring closure from cyclodecadiene Q or by photochemical electrocyclic ring closure from cyclodecadiene R. Draw the structures of Q and R, and indicate the stereochemistry of the process by which each reaction occurs.arrow_forwardCompounds A and B are isomers having molecular formula C5H12. Heating A with Cl2 gives a single product of monohalogenation, whereas heating B under the same conditions forms three constitutional isomers. What are thestructures of A and B?arrow_forward
- The two stereoisomers of cinnamic acid (C6H5CH=CHCO2H) each give a different product on reaction with 1,3-butadiene. Write an equation for each reaction.arrow_forwardCompound A, C9H16 reacts with 1 molar equivalent(s) of hydrogen on catalytic hydrogenation. A undergoes reaction with ozone, followed by Zn treatment. What is the structure for A?arrow_forward4. Compound A has the formula C 8H 8. It reacts rapidly with KMnO 4 to give CO 2 and a carboxylic acid, B (C 7H 6O 2), but reacts with only 1 molar equivalent of H 2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H 2 are taken up and hydrocarbon C (C 8H 16) is produced. What are the structures of A, B, and C.arrow_forward
- Methyl acrylate (H2C=CHCO2CH3) reacts with 1,3-cyclopentadiene to give a mixture of two products. Write structural formulas for both and predict which one predominates.arrow_forwardA chemist allows some pure (2S,3R)-3-bromo-2,3-diphenylpentane to react with a solution of sodium ethoxide(NaOCH2 CH3) in ethanol. The products are two alkenes: A (cis-trans mixture) and B, a single pure isomer. Under the same conditions, the reaction of (2S,3S)-3-bromo-2,3-diphenylpentane gives two alkenes, A (cis-trans mixture) and C. Upon catalytic hydrogenation, all three of these alkenes (A, B, and C) give 2,3-diphenylpentane. Determine the structures of A, B, and C; give equations for their formation; and explain the stereospecificity of these reactions.arrow_forwardAs an example of a ver rare occurence, the following hydrocarbon reacts with two equivalents of butylithium to form a dianion of the formula [C8H6]2- . Propose a structure for this dianion and explain why this dianion forms so readily.arrow_forward
- Compound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forwardCompound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forward
