
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
1st Edition
ISBN: 9781111580704
Author: Kevin D. Dahm, Donald P. Visco
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.12, Problem 22P
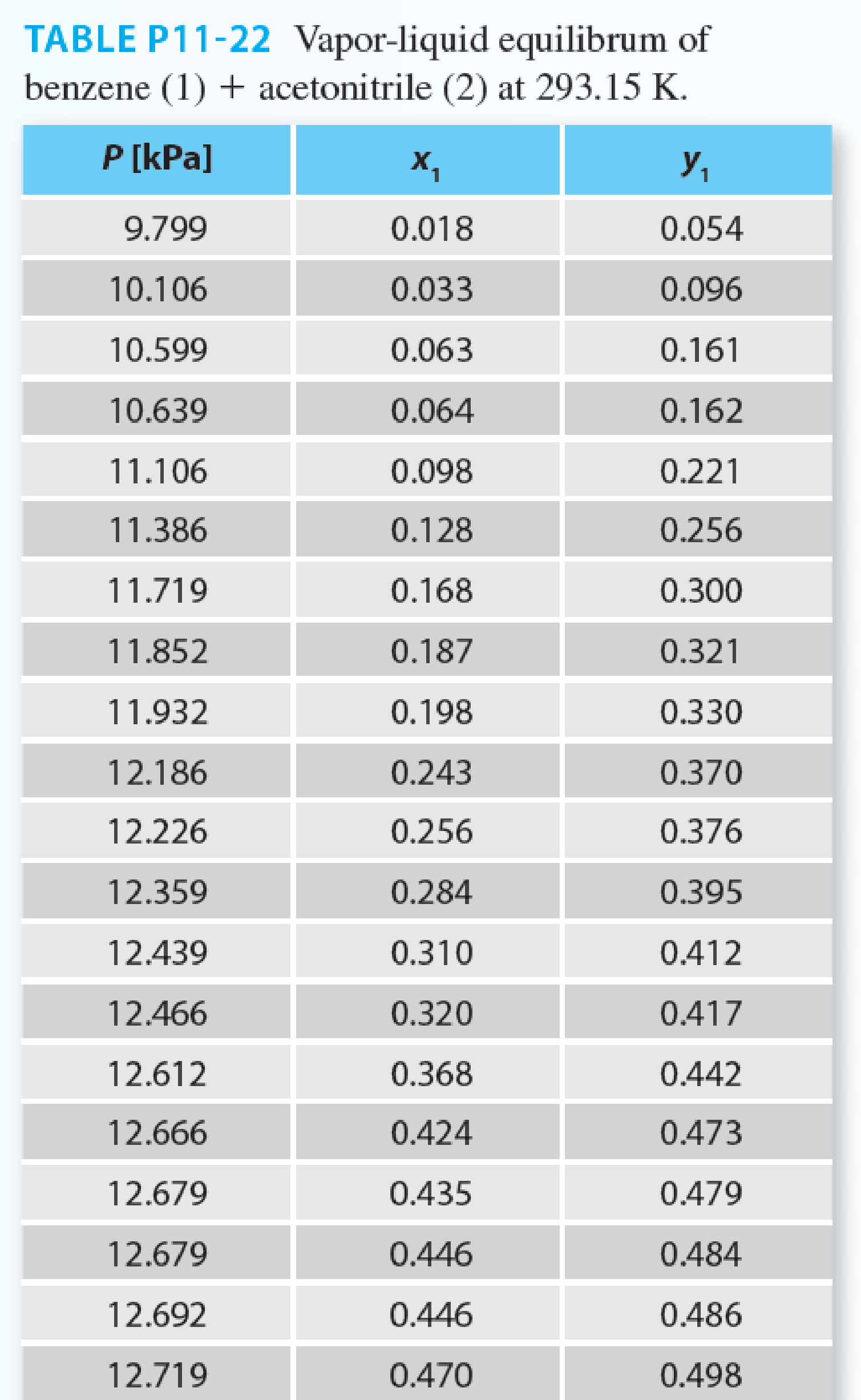
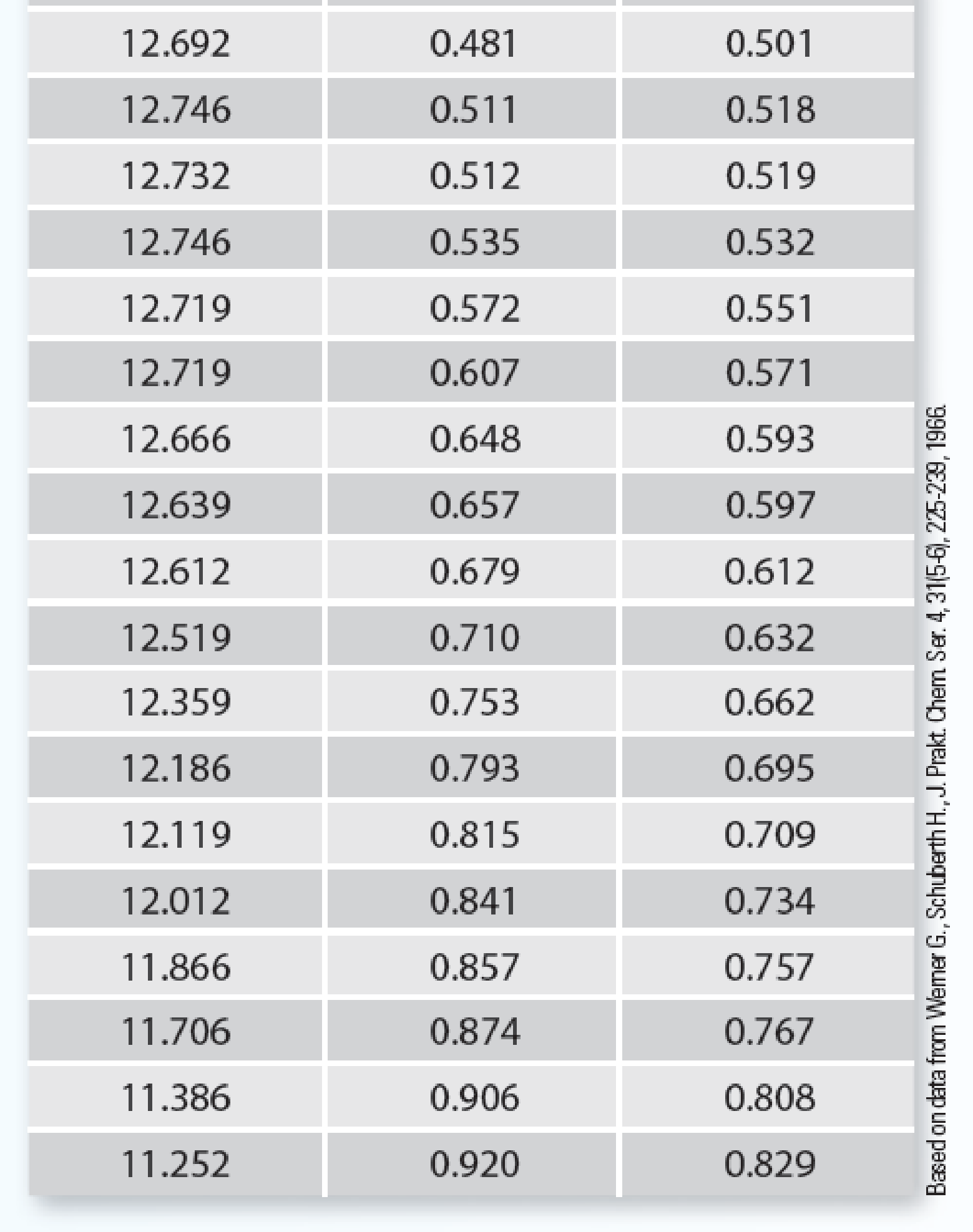
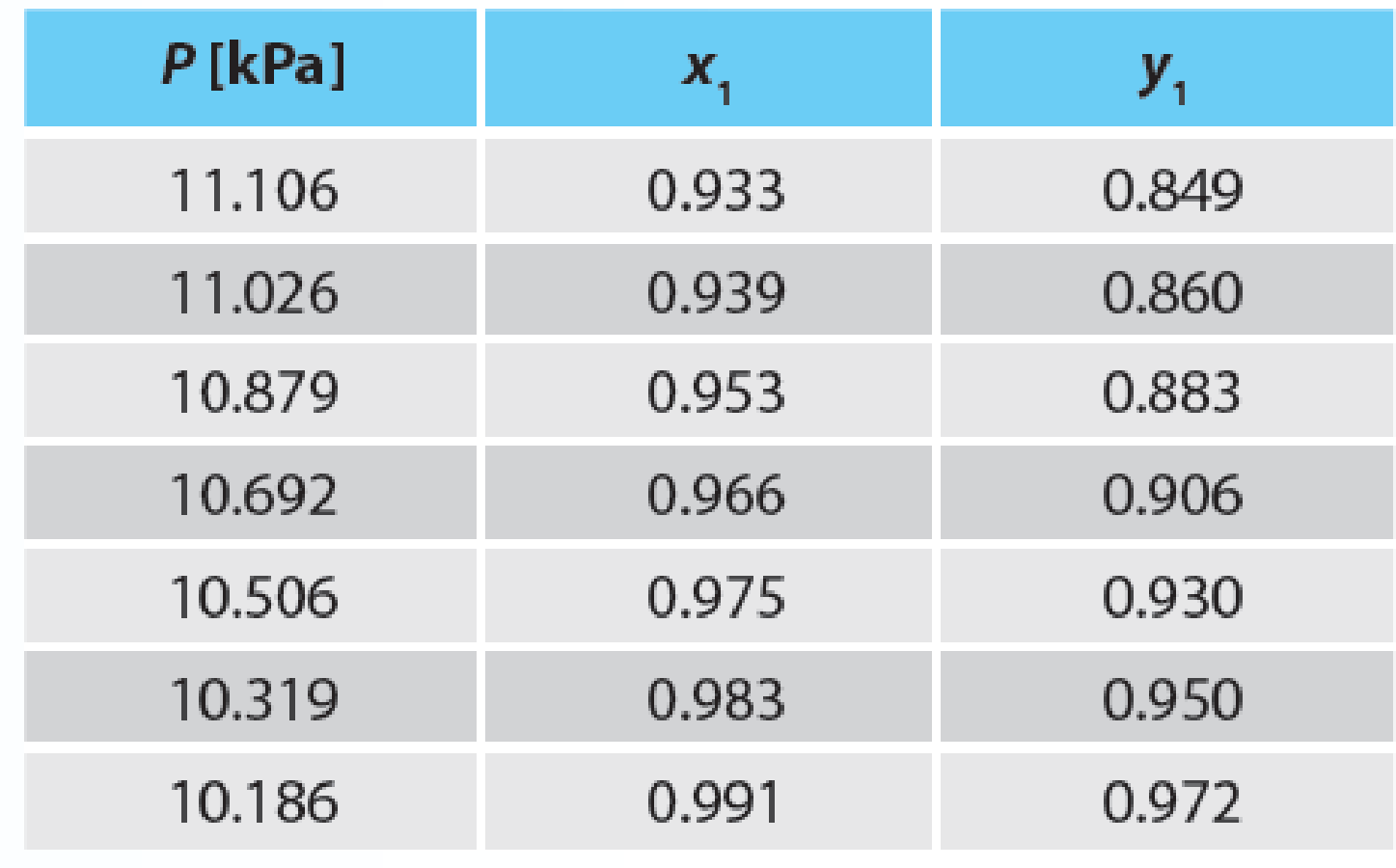
In the sizing of separation equipment, you need to know the vapor–liquid equilibrium for the benzene (1) + acetonitrile (2) system. You have data for this system at 293.15 K, but not at your desired temperature, which is 318.15 K. You also know this system has an azeotrope at 293.15 K at about 12.7 kPa and 53% benzene.
- A. Use the Wilson equation to fit all of the experimental data in Table P11-22 at 293.15 K.
- B. Use the Wilson parameters determined from part (A) to predict the system behavior at 318.15 K.
- C. Now that you have predicted the behavior (part (B)), how well does your prediction compare with the experimental data for this azeotrope at 318.15 K, which is P = 37.1 kPa and x1 = y1 = 0.52?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 11 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
Ch. 11.11 - The derivation of the expression for the natural...Ch. 11.11 - Derive the expression for the natural logarithm of...Ch. 11.11 - Prob. 3ECh. 11.11 - Given an equimolar binary mixture, calculate the...Ch. 11.11 - Prob. 5ECh. 11.11 - Prob. 6ECh. 11.11 - Calculate the van Laar parameters, L12 and L21,...Ch. 11.11 - Calculate the van Laar parameters using the...Ch. 11.11 - Explain why the integral test for thermodynamic...Ch. 11.11 - Prob. 10E
Ch. 11.12 - A binary liquid containing mostly component 2 is...Ch. 11.12 - Provide an estimate of the composition of N2...Ch. 11.12 - Resolve Example Problem 11.1, but now include the...Ch. 11.12 - A liquid mixture of 20% by mole benzene and the...Ch. 11.12 - You are interested in finding the pressure at...Ch. 11.12 - For a chloroform (1) + ethanol (2) system at 55C,...Ch. 11.12 - You are interested in finding the pressure at...Ch. 11.12 - The azeotrope of a binary mixture, being an...Ch. 11.12 - Consider the experimental data in Table P11-21 for...Ch. 11.12 - In the sizing of separation equipment, you need to...Ch. 11.12 - You are interested in evaluating how well you can...Ch. 11.12 - Compare the van Laar predictions if using...Ch. 11.12 - You desire to flash 20 moles/min of a liquid...Ch. 11.12 - Your company needs to evaluate the separation of...Ch. 11.12 - In a process you need to evaluate the flash...Ch. 11.12 - You desire to flash separate 10 mol/s of an...Ch. 11.12 - In a process analysis application, you are working...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Homogeneous and Heterogeneous Equilibrium - Chemical Equilibrium - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=8V9ozZSKl9E;License: Standard YouTube License, CC-BY