
Concept explainers
(a)
Interpretation:
The resultant product obtained from the metathesis of the alkene
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(b)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
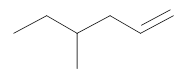
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(c)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
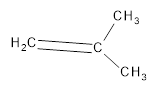
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(d)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
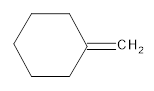
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry (8th Edition)
- What two sets of reagents (each consisting of a carbonyl compound and phosphonium ylide) can be used for the synthesis of each of the following alkenes?arrow_forwardWhat is the major organic product obtained from the following reaction?arrow_forwardWhat are the products for the following reactions? Be aware of alkene stereochemistryarrow_forward
- What is the major product obtained from the reaction of HBr with each of the following?arrow_forwardProvide the alkene needed to synthesize each of the compounds given by oxymercuration– demercuration. Provide the alkene needed to synthesize each of the compounds given by hydroboration- oxidation.arrow_forwardGive the alkene and reagent that are needed to synthesize each of the following compoundsarrow_forward
- How could the following compound be prepared using an alkene as one of the starting materials? You can use the suitable reactives for this reaction.arrow_forwardWhat two sets of reagents (each consisting of a carbonyl compound and phosphonium ylide) can be used for the synthesis of each of the following alkenes? What alkyl halide is required to prepare each of the phosphonium ylides? What is the best set of reagents to use for the synthesis?arrow_forwardWrite down the major products or the other reactants and or the reaction conditions for the following reactions showing stereochemistry whenever appropriatearrow_forward
- One possible way of determining the identity of an alkene, is to let itundergo an oxidative cleavage reaction in the presence of hot basicpotassium permanganate. You are given two containers said to containdifferent alkenes. Container A is marked as cis / trans‐2‐butene andcontainer B as 2‐methyl‐1‐butene. Explain by referring to the formation ofproducts, how you would verify the identity of the alkenes.arrow_forwardWhat starting material is required in order to synthesize each of the following compounds by ring-closing metathesis?arrow_forwardWhat is the major organic product obtained from the following reactions ?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





