
Concept explainers
(a)
Interpretation:
To analyze whether the given statement- There are two classes of
Concept Introduction:
Hydrocarbons consists of carbon atoms and hydrogen atoms. They are further classified as saturated or unsaturated on the basis that the hydrocarbon has all single bonds, or it involves double or triple bond also.
The hydrocarbon that has all single bond is called as
The hydrocarbon that has at least one double bond is called as alkene wit the general formula
The hydrocarbon that has at least one triple bond is called as alkyne wit the general formula
(a)
Answer to Problem 12.11P
There are two classes of unsaturated hydrocarbons-alkenes and alkynes. Thus, the statement is true.
Explanation of Solution
Hydrocarbons consists of carbon atoms and hydrogen atoms. They are further classified as saturated or unsaturated on the basis that the hydrocarbon has all single bonds or it involves double or triple bond also.
The hydrocarbon that has all single bond is called as alkane with the general formula
Example- Ethane

The hydrocarbon that has at least one double bond is called as alkene with the general formula
Example- Ethene
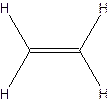
The hydrocarbon that has at least one triple bond is called as alkyne with the general formula
Example- Ethyene

Since all the bonds in alkenes and alkynes are not single bonds, they are unsaturated hydrocarbons
(b)
Interpretation:
To analyze whether the given statement- The bulk of the ethylene used by the chemical industry worldwide is obtained from renewable resources , is true or false.
Concept Introduction:
Ethylene is an unsaturated hydrocarbon, with the formula
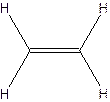
Renewable sources are those sources which when used once can be generated again.e.g. solar energy is a renewable source of energy.
Non-renewable sourcesare those sources which when used once are generated after millions of years e.g. coal and petroleum are non-renewable sources of energy.
The bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources. Thus, the statement is true.
There are several methods by which ethylene is extracted
- Steam Cracking of-
- Ethane and propane from natural gas or crude oil
- Naptha from crude oil
- Catalytic cracking of gas oil from crude oil
Crude oil and natural gas both are non-renewable sources. Therefore, the bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources
(c)
Interpretation:
To analyze whether the give statement- Ethylene and acetylene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
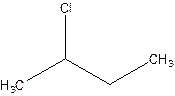
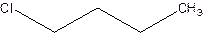
2-Chlorobutane 1-Chlorobutane
Ethylene and acetylene are not constitutional isomers. Thus, the statement is false.
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for ethylene
The structural formula for ethylene-
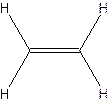
The molecular formula for acetylene
The structural formula for acetylene-
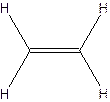
Since ethylene and acetylene neither have same molecular formula nor structural formula. So they are not constitutional formula.
(d)
Interpretation:
To analyze whether the give statement- Cyclohexane and 1-hexene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
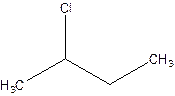
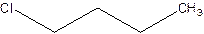
2-Chlorobutane 1-Chlorobutane
Cyclohexane and 1-hexene are constitutional isomers. Thus, the statement is true.
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for Cyclohexane
The structural formula for Cyclohexane -
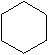
Cyclohexane
The molecular formula for 1-hexene
The structural formula for 1-hexene -

1-hexene
Since, cyclohexane and 1-hexene have same molecular formula and structural formula so, they are constitutional formula.
(b)
Answer to Problem 12.11P
The bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources. Thus, the statement is true.
Explanation of Solution
There are several methods by which ethylene is extracted
- Steam Cracking of-
- Ethane and propane from natural gas or crude oil
- Naptha from crude oil
- Catalytic cracking of gas oil from crude oil
Crude oil and natural gas both are non-renewable sources. Therefore, the bulk of the ethylene used by the chemical industry worldwide is obtained from non-renewable resources
(c)
Interpretation:
To analyze whether the give statement- Ethylene and acetylene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
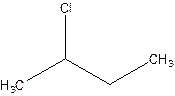

2-Chlorobutane 1-Chlorobutane
(c)
Answer to Problem 12.11P
Ethylene and acetylene are not constitutional isomers. Thus, the statement is false.
Explanation of Solution
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for ethylene
The structural formula for ethylene-
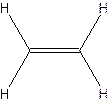
The molecular formula for acetylene
The structural formula for acetylene-
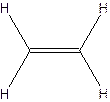
Since ethylene and acetylene neither have same molecular formula nor structural formula. So they are not constitutional formula.
(d)
Interpretation:
To analyze whether the give statement- Cyclohexane and 1-hexene are constitutional isomers, is true or false.
Concept Introduction:
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula. For example- 2-Chloro butane and 1-Chloro butane are constitutional isomers.
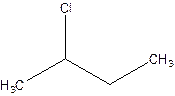

2-Chlorobutane 1-Chlorobutane
(d)
Answer to Problem 12.11P
Cyclohexane and 1-hexene are constitutional isomers. Thus, the statement is true.
Explanation of Solution
There are compounds which have same constituents but differ in the arrangement of atoms in space. The constitutional isomers are those compounds that have same molecular formula but different structural formula.
The molecular formula for Cyclohexane
The structural formula for Cyclohexane -

Cyclohexane
The molecular formula for 1-hexene
The structural formula for 1-hexene -

1-hexene
Since, cyclohexane and 1-hexene have same molecular formula and structural formula so, they are constitutional formula.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- . Alkenes and alkynes are characterized by their ability to undergo rapid, complete reactions, by which other atoms attach themselves to the carbon atoms of the double or triple bond.arrow_forwardWhat is meant by the term “unsaturated hydrocarbon”? What structural feature characterizes unsaturated hydrocarbons?arrow_forwardSummarize the nomenclature rules for alkanes, alkenes, alkynes, and aromatic compounds. Correct the following false statements regarding nomenclature of hydrocarbons. a. The root name for a hydrocarbon is based on the shortest continuous chain of carbon atoms. b. The suffix used to name all hydrocarbons is -ane. c. Substituent groups are numbered so as to give the largest numbers possible. d. No number is required to indicate the positions of double or triple bonds in alkenes and alkynes. e. Substituent groups get the lowest number possible in alkenes and alkynes. f. The ortho- term in aromatic hydrocarbons indicates the presence of two substituent groups bonded to carbon- 1 and carbon-3 in benzene.arrow_forward
- Select those compounds that can be correctly called unsaturated and classify each one as an alkene or an alkyne: a.CH3CH2CH3f. b.CH3CH=CHCH3g. c.h.CH2=CHCH2CH3 d.i. e.arrow_forwardWhat is an isomer having the formula C6H12 ?Without using 2-hexenearrow_forwardTrue or false: 2,2-dimethylpropane is a structural isomer of n-pentanearrow_forward
- Give the complete IUPAC names of the alkanes and cycloalkanes below. B. Draw the structures and give the IUPAC name of the five constitutional isomers of2-methylhexane.arrow_forwardAnswer true or false. (a) Alkenes and alkynes are nonpolar molecules. (b) The physical properties of alkenes are similar to those of alkanes of the same carbon skeletons. (c) Alkenes that are liquid at room temperature are insoluble in water and when added to water, will float on water.arrow_forwardGive the molecular formula of a hydrocarbon containingfive carbon atoms that is (a) an alkane, (b) a cycloalkane,(c) an alkene, (d) an alkyne.arrow_forward
- Hi, here's my question. Write the condensed structural formulas for two alkenes and one alkyne that all have the molecular formula C6H10.arrow_forwardGive the molecular formula of a hydrocarbon containingsix carbon atoms that is (a) a cyclic alkane, (b) a cyclicalkene, (c) a linear alkyne, (d) an aromatic hydrocarbon.arrow_forwardWhich of the following statements about alkanes, alkenes and alkynes is FALSE?A. Alkanes can be solids, liquids or gases.B. Alkenes are always gases in state at room temperature.C. Alkanes are more reactive to halogenation compared to alkenes.D. Alkynes exhibit the greatest electron density among hydrocarbonsarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





