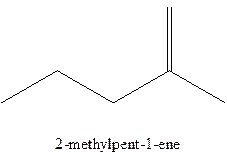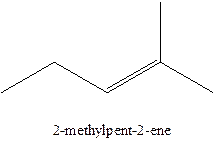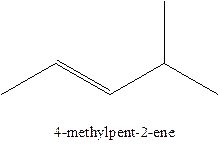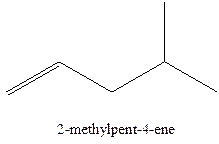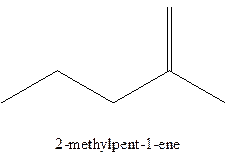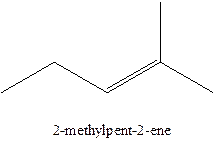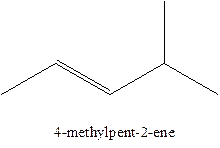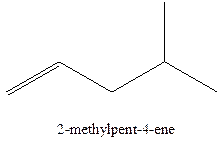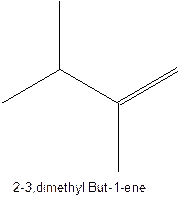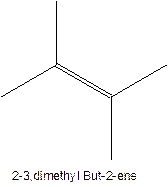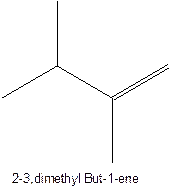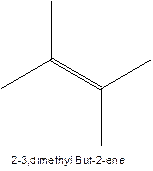Concept explainers
(a)
Interpretation:
To name and draw structural formula for
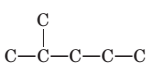
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
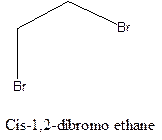
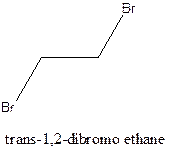
Answer to Problem 12.16P
The alkenes with the molecular formula
Explanation of Solution
The longest chain for the given skeleton has 5 carbons therefore the root name prop- is used here. The alkenes with the molecular formula
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(b)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula
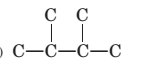
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
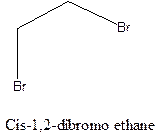
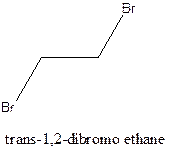
Answer to Problem 12.16P
The alkenes with the molecular formula
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkenes with the molecular formula
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(c)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula
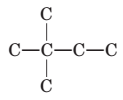
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
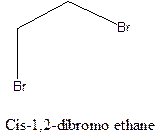
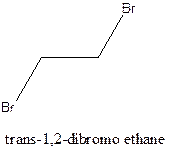
Answer to Problem 12.16P
The alkene with the molecular formula
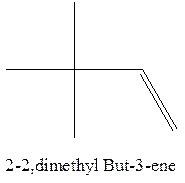
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkene with the molecular formula
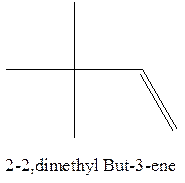
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(c)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula

Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
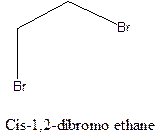
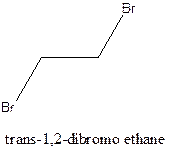
Answer to Problem 12.16P
The alkene with the molecular formula
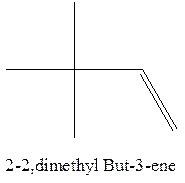
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkene with the molecular formula
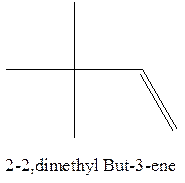
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- Which of the following cycloalkanes could show geometric isomerism? For each that could, draw structural formulas, and name both the cis- and the trans- isomers. a. c. b. d.arrow_forward. Alkenes and alkynes are characterized by their ability to undergo rapid, complete reactions, by which other atoms attach themselves to the carbon atoms of the double or triple bond.arrow_forwardDistinguish between isomerism and resonance. Distinguish between structural and geometric isomerism. When writing the various structural isomers, the most difficult task is identifying which are different isomers and which are identical to a previously written structurethat is, which are compounds that differ only by the rotation of a carbon single bond. How do you distinguish between structural isomers and those that are identical? Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C4H8. Another common feature of alkenes and cycloalkanes is that both have restricted rotation about one or more bonds in the compound, so both can exhibit cis- trans isomerism. What is required for an alkene or cycloalkane to exhibit cis-trans isomerism? Explain the difference between cis and trans isomers. Alcohols and ethers are structural isomers of each other, as are aldehydes and ketones. Give an example of each to illustrate. Which functional group in Table 21-4 can be structural isomers of carboxylic acids? What is optical isomerism? What do you look for to determine whether an organic compound exhibits optical isomerism? 1-Bromo-1-chloroethane is optically active whereas 1-bromo-2-chloroethane is not optically active. Explain.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning