
Concept explainers
12-23 Draw a structural formula for each compound.
- 3-Chloropropene
- 3-Methylcyclohexene
- 1,2-Dimethylcyclohexene
- /runs-3,4-Dimethyl-3-heptene
- Cydopropene
- 3-Hexyne
(a)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space.
In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 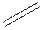 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P
Explanation of Solution
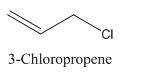
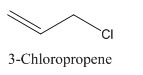
3-Chloropropene.
Carbon atoms in the longest chain are 3.
-ene means a double bond is present at Carbon 1.
Chlorine at Carbon 3.
Structure.
(b)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 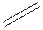 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P
Explanation of Solution
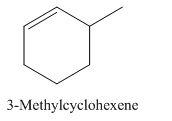
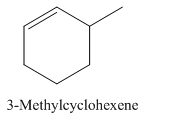
3-Methylcyclohexene.
Carbons in longest chain=6.
Cyclo means a cyclic hydrocarbon.
-ene means a double bond is present at Carbon 1.
Methyl at Carbon 3.
Structure.
(c)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 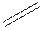 and triple bond as
and triple bond as  The carbon atoms are represented as vertices and end of lines.
The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P
Explanation of Solution
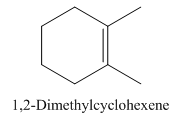
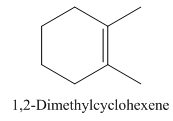
1,2-Dimethylcyclohexene.
Carbons in longest chain=6.
Cyclo means a cyclic hydrocarbon.
-ene means a double bond is present at Carbon 1.
Methyl at Carbon 1 and 2.
Structure.
(d)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 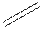 and triple bond as
and triple bond as  The carbon atoms are represented as vertices and end of lines.
The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P
Explanation of Solution
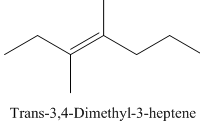
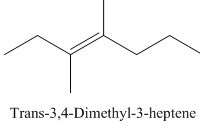
Trans-3,4-Dimethyl-3-heptene.
Carbons in longest chain=7.
-ene means a double bond is present at Carbon3.
Methyl at Carbon 3 and 4.
Trans isomer that means the two methyl are opposite in direction.
Structure.
(e)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 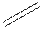 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P

Explanation of Solution
Cyclopropene.
Carbons in longest chain=3.
-ene means a double bond is present at Carbon1.
Cyclo means a cyclic hydrocarbon.
Structure.

(f)
Interpretation:
To draw the structural formula for the compound.
Concept Introduction:
Structural formula is the spatial arrangement of atoms of a molecule in space. In the condensed formula is a line-angle formula, the symbol for carbon and hydrogen atoms are not shown. Only carbon-carbon bonds are shown using lines. Single bonds are shown as  double bond as
double bond as 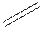 and triple bond as
and triple bond as  . The carbon atoms are represented as vertices and end of lines.
. The carbon atoms are represented as vertices and end of lines.
Answer to Problem 12.23P
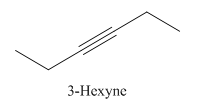
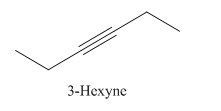
Explanation of Solution
3-Hexyne.
Carbons in longest chain=6.
-yne means a triple bond is present at Carbon3.
Structure.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- 12-45 Draw a structural formula for the product of each reaction. 1-Methylcyclohexene + Br2 1,2-Dimethylcyclopentene + Cl2arrow_forward12-27 Explain why each name is incorrect and then write a correct name. 2-Ethyl-l-propene 5-lsopropylcyclohexene 4-Methyl-4-hexene 2-sec-Butyl-l-butene 6,6-Dimethylcyclohexene 2-Ethyl-2-hexenearrow_forward12-59 Show how to convert 1-butene to these compounds, (a) Butane(b) 2-Butanol (c) 2-Bromobutane(d) 1,2-Dibromobutanearrow_forward
- 12-58 Show how to convert ethylene to these compounds (a) Ethane(b) Ethanol (c) Bromoethane(d) 1,2-Dibromoethane (e) Chloroethanearrow_forward12-29 Which of these alkenes show cis-trans isomerism? For each that does, draw structural formulas for both isomers. (a) 1-Hexene(b)2-Hexene (c) 3-Hexene(d)2-Methyl-2-hexene (e) 3-Methyl-2-hexene(f)2,3-Dimethyl-2-hexenearrow_forward/3-Ocimene, a triene found in the fragrance of cotton blossoms and several essential oils, has the 1UPAC name czs-3,7-dimethyl-l,3,6-octatriene. (Cis refers to the configuration of the double bond between carbons 3 and 4, the only double bond in this molecule about which cis-trans isomerism is possible.) Draw a structural formula for /3-ocimene.arrow_forward
- 12-15 Name and draw structural formulas for all alkenes with the molecular formula C5H1U. As you draw these alkenes, remember that cis and trans isomers are different compounds and must be counted separately.arrow_forward11-38 Why is equatorial methylcyclohexane more stable than axial methylcyclohexane?arrow_forward12-40 Define alkene addition reaction. Write an equation for an addition reaction of propene.arrow_forward
- Draw a structural formula for an alkene with the indicated molecular formula that gives the compound shown as the major product. Note that more than one alkene may give the same compound as the major product. CHa | C5Hw + H,O CH3CCH2CH3 OH CHa I C5H10 + Br2 » CH3CHCHCH, Br Br 7H12 + HC1 oearrow_forward11-52 Draw structural formulas for these haloalkanes Bromomethane Chlorocyclohexane 1,2-Dibromoethane 2-Chloro-2-methylpropane Dichlorodilluoromethane (Freon-12)arrow_forwardDraw at least two structural formulas for each of the following. (Several constitutional isomers are possible for each part.) An alkene with six carbons A cycloalkene with six carbons An alkyne with six carbons An aromatic hydrocarbon with eight carbonsarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


