
Concept explainers
Draw the products formed when A is treated with each reagent: (a)
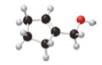
A
(a)
Interpretation: The product formed when A is treated with
Concept introduction: The addition of
Answer to Problem 12.29P
The product formed when A is treated with
Explanation of Solution
The given molecule is,
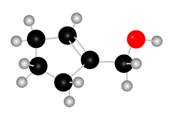
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of A is,
Figure 2
When A is treated with
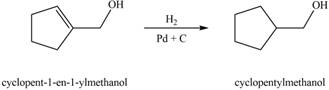
Figure 3
(b)
Interpretation: The product formed when A is treated with
Concept introduction: In presence of peroxide alkene is oxidized to epoxide this is known as epoxidation. The weak pi bond of alkene and weak
Answer to Problem 12.29P
The product formed when A is treated with
Explanation of Solution
The given molecule is,
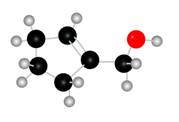
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the A is,

Figure 2
In presence of
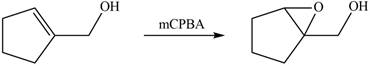
Figure 4
(c)
Interpretation: The product formed when A is treated with
Concept introduction: Alcohols are oxidized to different carbonyl compounds depending upon the reagents and alcohol used. In presence of strong oxidizing reagents such as
Answer to Problem 12.29P
The product formed when A is treated with
Explanation of Solution
The given molecule is,
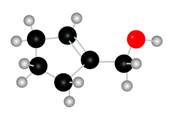
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the A is,
Figure 2
The chemical reaction of A with
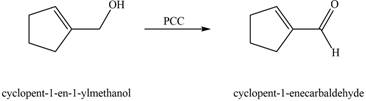
Figure 5
The primary alcohol is oxidized to aldehyde in presence of mild oxidizing reagent
(d)
Interpretation: The product formed when A is treated with
Concept introduction: Alcohols are oxidized to different carbonyl compounds depending upon the reagents and alcohol used. In presence of strong oxidizing reagents such as
Answer to Problem 12.29P
The product formed when A is treated with
Explanation of Solution
The given molecule is

Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the A is,

Figure 2
When A is treated with
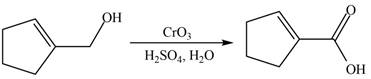
Figure 6
The primary alcohol is oxidized to carboxylic acid in presence of strong oxidizing reagent
(e)
Interpretation: The product of formed when A is treated with Sharpless reagent
Concept introduction: Sharpless epoxidation involves the oxidation of double bond between carbon atoms to epoxide. This oxidation occurs only in allylic alcohol. This is an enantioselective oxidation, which means predominantly one enantiomer is formed. Sharpless reagents are
Answer to Problem 12.29P
The product of formed when A is treated with Sharpless reagent
Explanation of Solution
The given molecule is
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the A is,
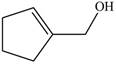
Figure 2
When epoxidation is done using
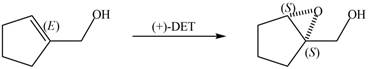
Figure 7
(a) The product formed when A is treated with
(b) The product formed when A is treated with
(c) The product formed when A is treated with
(d) The product formed when A is treated with
(e) The product of formed when A is treated with Sharpless reagent
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
Additional Science Textbook Solutions
General, Organic, & Biological Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Organic Chemistry - Standalone book
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
General Chemistry: Principles and Modern Applications (11th Edition)
Introduction to Chemistry
- Draw the products formed when A is treated with each reagent: (a) H2 + Pd-C; (b) mCPBA; (c) PCC; (d) CrO3, H2SO4, H2O; (e) Sharpless reagent with (+)-DET.arrow_forwardWhich of the following is not a monodentate ligand ? Select one: a. OH- b. H2NCH2CH2NH2 c. H2O d. CN-arrow_forwardDraw the product when attached compound is treated with either (CH3)2CuLi, followed by H2O, or HC≡CLi, followed by H2O.arrow_forward
- Draw the products 1. (S)-2-chlorobutane and sodium acetate in DMSO 2. 1-bromopropane and methylamine in acetonitrile.arrow_forwarda) Which reaction yields hexan-1-ol?arrow_forwardDraw the product(s) formed when A is treated with each reagent.a. NaBH4, CH3OH b. LiAlH4, then H2O c. Ag2O, NH4OH d. CrO3, H2SO4, H2O e. PCCarrow_forward
- Thioglycolic acid, HSCH2CO2H, a substance used in depilatory agents (hair removers) has pKa = 3.42. What is the percent dissociation of thioglycolic acid in a buffer solution at pH = 3.0?arrow_forward2. Draw the reaction and resulting product when 1-heptyne is trrated with excess HBr? Draw the reaction and resulting product when 1-ethyne in H20 is treated with H2SO4 + HgSO4?arrow_forwardWhy does the alpha hydrogen to a ketone have a lower pka value than the alpha h to an alkene. Draw a picture to explain ur a waysarrow_forward
