
Concept explainers
12-42 Complete these equations.
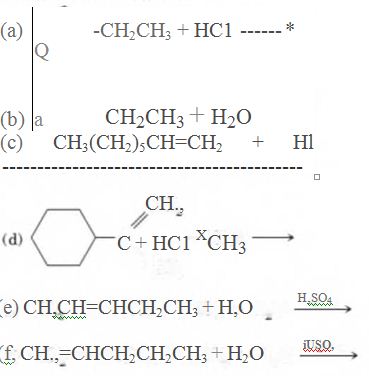
(a)
Interpretation:
Complete the below equation:
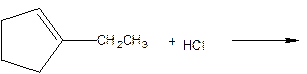
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and Cl- ions. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
Explanation of Solution
As per the given equation, the reactant is a cyclic alkene to which HCl is added. And the characteristics reactions given by the alkenes are addition reactions in which the addition takes place at the double bond. The addition follows the Markovnikov’s rule.
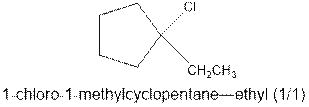
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is Cl-. On numbering the carbon atoms, the structure is.
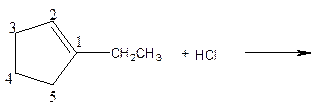
Now as per the rule, the hydrogen in the above equation attaches to carbon 2 as carbon 2 of the double bond has one hydrogen attached to it. The Cl- will attached to carbon 1 as it has no carbon attached to it. Thus, the complete reaction is:
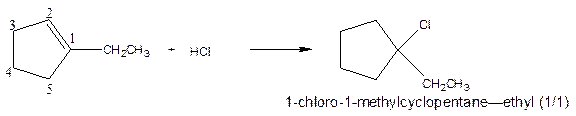
(b)
Interpretation:
Complete the below equation:
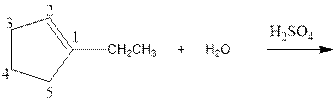
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
Explanation of Solution
As per the given equation, the reactant is a cyclic alkene to which H2 O is added. And the characteristics reactions given by the alkenes are addition reactions in which the addition takes place at the double bond. The addition follows the Markovnikov’s rule.
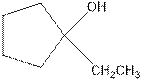
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the carbon atoms, the structure is.
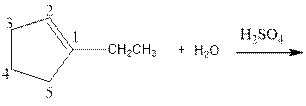
Now as per the rule, the hydrogen in the above equation attaches to carbon 2 as carbon 2 of the double bond has one hydrogen attached to it. The OH- will attached to carbon 1 as it has no carbon attached to it. Thus, the complete reaction is:
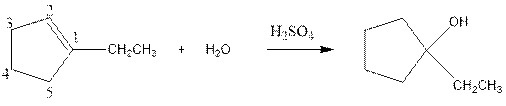
(c)
Interpretation:
Complete the below equation:
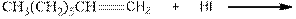
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and I- ions.
Answer to Problem 12.42P
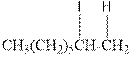
Explanation of Solution
As per the above equation, the reactant is an alkene to which HI is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is I-. On numbering the double bonded carbons of the alkene, the structure is.
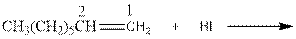
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The I- will attached to carbon 2 as it has only one carbon attached to it. Thus, the complete reaction is:
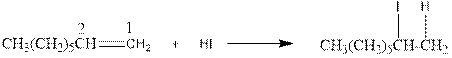
(d)
Interpretation:
Complete the below equation:

Concept introduction:
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it.
Answer to Problem 12.42P
Explanation of Solution
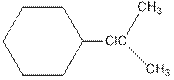
As per the above equation, the reactant is an alkene to which HCl is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is Cl-. On numbering the double bonded carbons of the alkene, the structure is.
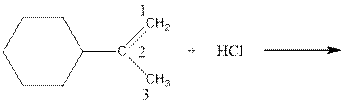
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The Cl- will attached to carbon 2 as it has only no carbon attached to it. Thus, the complete reaction is:

(e)
Interpretation:
Complete the below equation:
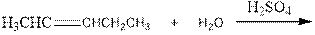
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
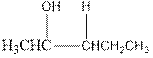
Explanation of Solution
As per the above equation, the reactant is an alkene to which H2 O is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the alkene, the structure is.
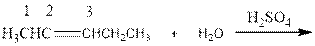
Now the below product will form where hydrogen will attach to the 2nd carbon to form more suitable product:
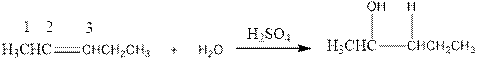
(f)
Interpretation:
Complete the below equation:
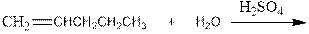
Concept introduction:
During the hydration of alkene the unsaturated alkene converted into saturated alkane with the addition of H+ and OH- ions in the presence of H2 SO4. This reaction is carried out in the presence of acid therefore it is called acid catalyzed hydration of alkene.
Answer to Problem 12.42P
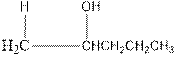
Explanation of Solution
As per the above equation, the reactant is an alkene to which H2 O is added. We also know that the characteristics reactions given by alkenes are addition reactions in which the addition takes place at the double bond. This addition follows the Markovnikov’s rule.
As per the Markovnikov’s rule, the hydrogen acid is added to the carbon of a double bond containing higher number of hydrogens in it. The halogen part is added to the carbon in the double bond containing fewer number of hydrogens in it. Here, the positive part is H+ and the halogen part is OH-. On numbering the alkene, the structure is.
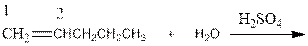
Now as per the rule, the hydrogen in the above equation attaches to carbon 1 as carbon 1 of the double bond has two hydrogen attached to it. The OH- will attached to carbon 2 as it has only one carbon attached to it. Thus, the complete reaction is:
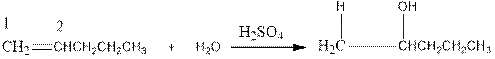
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- 11-58 (Chemical Connections 11A) How many rings in te trodotoxin contain only carbon atoms? How many contain nitrogen atoms? How many contain two oxygen atoms?arrow_forward13-39 (Chemical Connections 13B) What is a carcinogen What kind of carcinogen is found in cigarette smoke?arrow_forward12-61 (Chemical Connections 12A) What is one function of ethylene as a plant growth regulator?arrow_forward
- As stated in Section 11-9, the wax found in apple skins is an unbranched alkane with the molecular formula C^H^. Explain how the presence of this alkane in apple skins prevents the loss of moisture from within the apple.arrow_forward13-38 (Chemical Connections 13A) What is meant by the term biodegradable?arrow_forwardThe heat of combustion of methane, a component of natural gas, is 212 kcal/mol. That of propane, a component of LP gas, is 530 kcal/mol. On a gram- for-gram basis, which hydrocarbon is the better source of heat energy?arrow_forward
- (Chemical Connections 11C) What are HFCs and HCFCs? How does their use in refrigeration systems prevent the environmental problems associated with the use of Freons?arrow_forwardAmong the ingredients listed in one commercial foam shaving gel are isobutane and isopentane. Write the 1UPAC name of each hydrocarbon. Why are these two hydrocarbons added to the formulation of the shaving gel?arrow_forward13-40 (Chemical Connections 130 In the absence of iodine in the diet, goiter develops. Explain why goiter is a regional disease.arrow_forward
- 10-27 What is meant by the term functional group?arrow_forward11-62(Chemical Connections 11C) What are Freons? Why were they considered ideal compounds to use as heat-transfer agents in refrigeration systems? Give structural formulas of two Freons used for this purpose.arrow_forward13-31 What structural features are common to vitamin E, BHT, and BHA (the three antioxidants presented in Section 13-40?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
