
Concept explainers
Indicate whether each of the following changes represents oxidation or reduction.
- a. FADH2 → FAD
- b. FMN → FMNH2
- c. Fe(III)SP → Fe(II)SP
- d. Cyt c1 (Fe3+) → cyt c1 (Fe2+)
(a)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
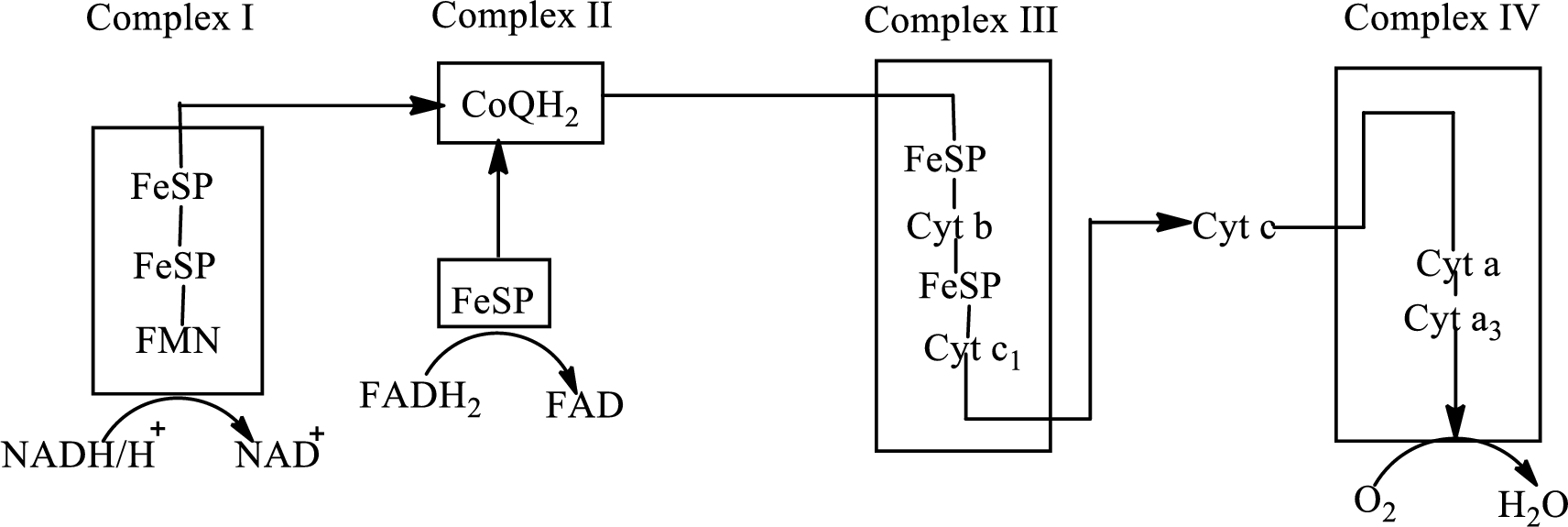
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
In the complex II, electrons are transferred from the
Here,
(b)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
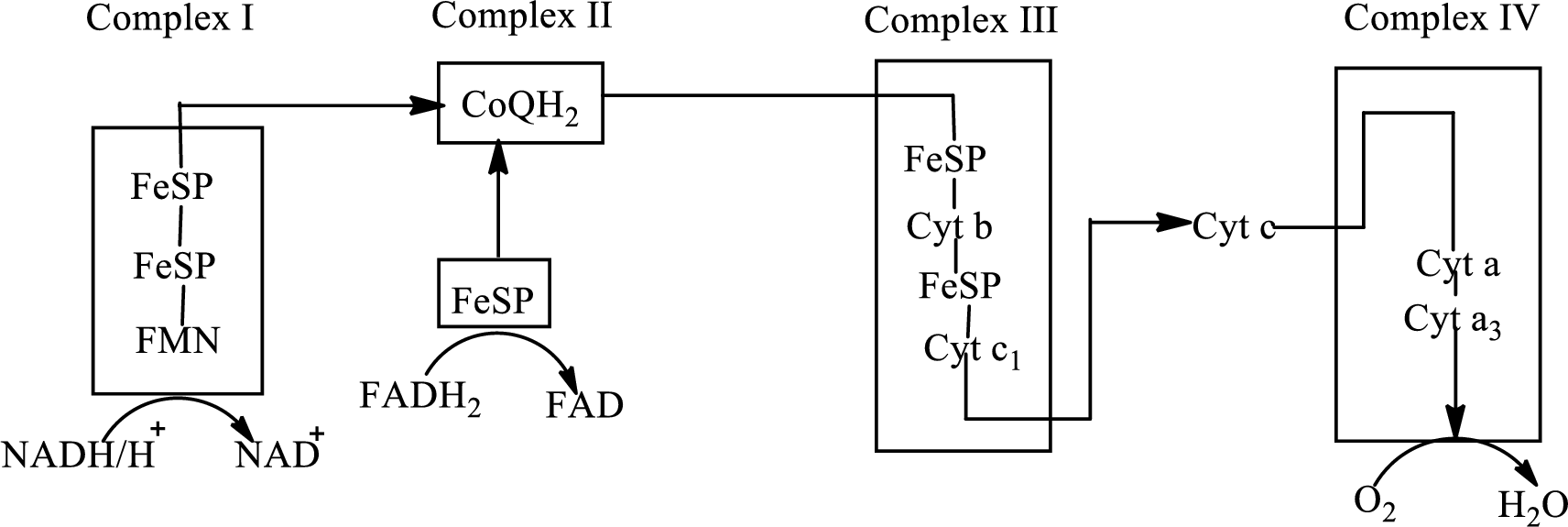
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Flavin mononucleotide is the structural component of the complex I of the electron transport chain. Flavin mononucleotide exists in two forms: FMN (oxidized form) and
Here, FMN gains electrons and hydrogen and leads to the formation of
(c)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
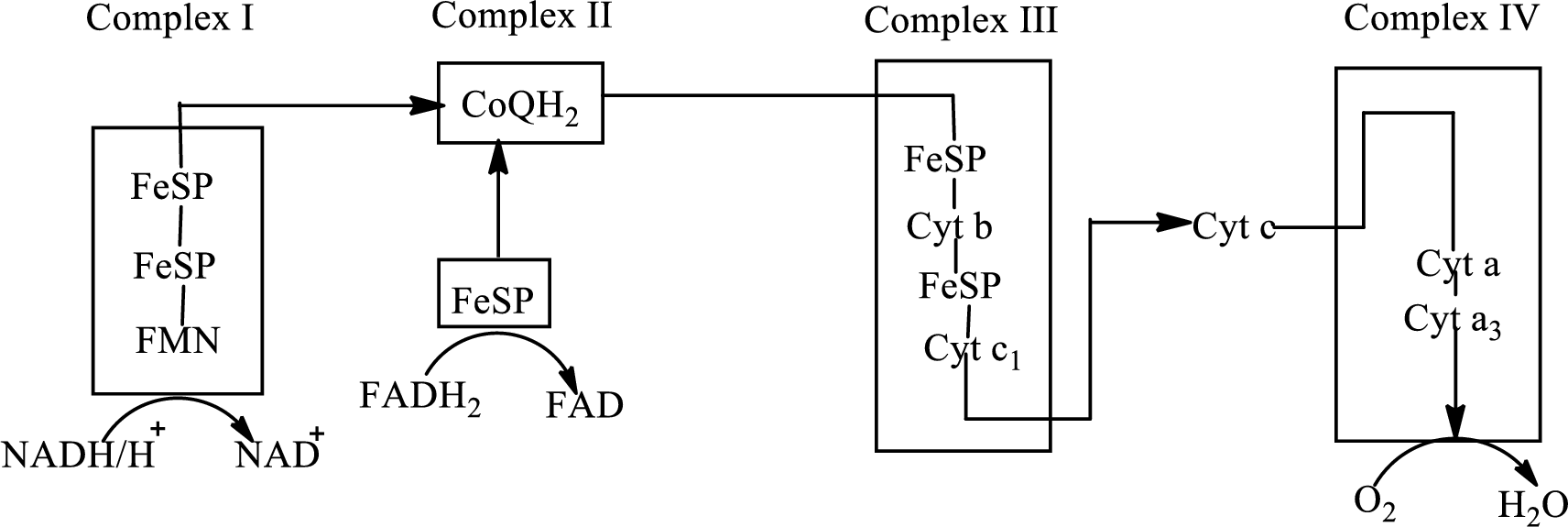
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Iron-sulfur proteins
(d)
Interpretation:
To indicate whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
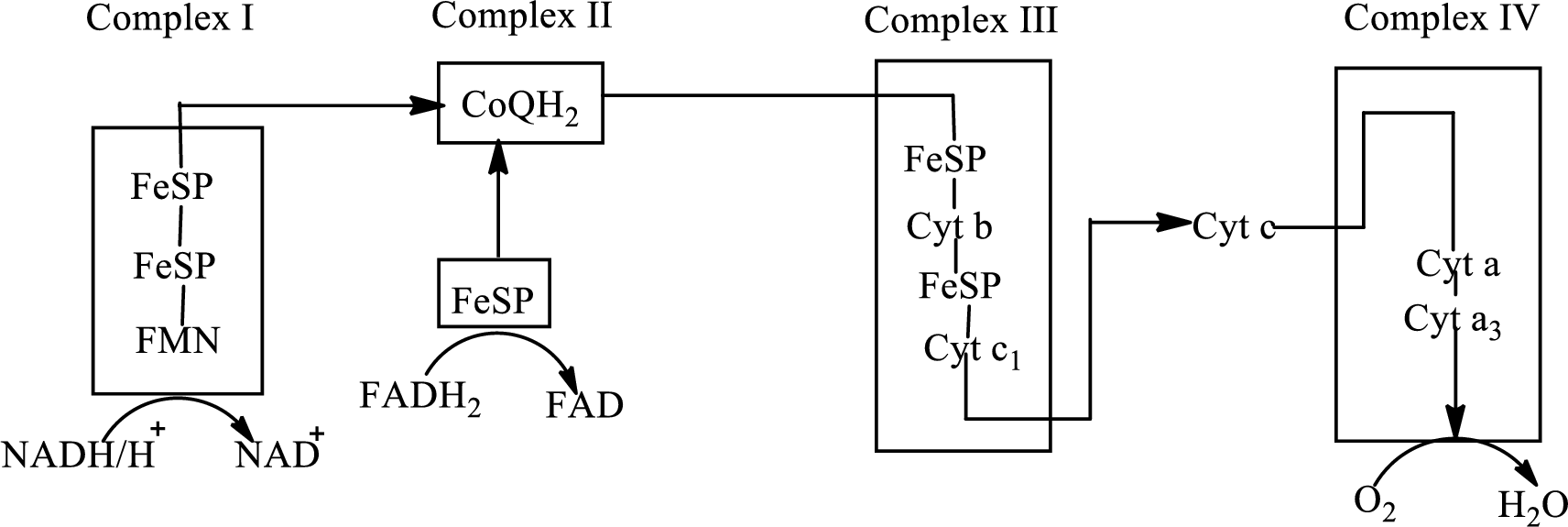
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is,
Here, A is oxidized form and AH is reduced form.
Answer to Problem 12.90EP
The change
Explanation of Solution
Cytochromes are a structural component of the complex III and consist of iron that changes its oxidation state from
Want to see more full solutions like this?
Chapter 12 Solutions
Organic And Biological Chemistry
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Chemistry & Chemical Reactivity
Chemistry
Chemistry: The Central Science (13th Edition)
Chemistry (7th Edition)
Chemistry & Chemical Reactivity
- Which nutrient provides energy in its most concentrated form?arrow_forwardHow many conjugated double bonds are there in a. FAD? b. FADH2?arrow_forward1.In the mitochondria, enzymes attached to the membrane use the ______________ to produce a ____________ difference and thus an electrical potential. Making the transfer of _______________ possible a. pH, flow of electrons, hydrogens b. pH, electron flow, oxygen c. electron flow, pH, hydrogens d. Electron flow, pH, Oxygen 2.Which of the following sugars can be a substrate for hexokinase? a.glucose b.fructose c.mannose d.all these e.none of thosearrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




