
Concept explainers
Interpretation:
The structural formulas and the IUPAC names for the
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.9E
The structural formulas and the IUPAC names of the
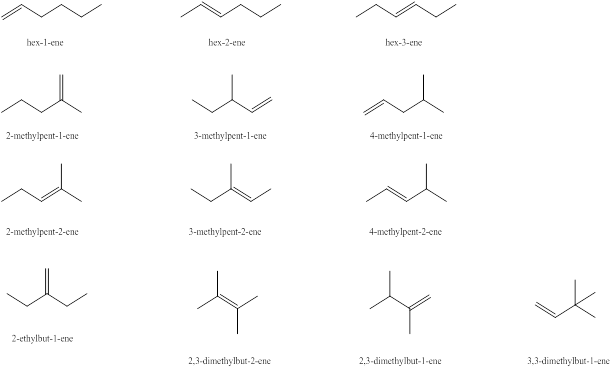
Explanation of Solution
The first alkene isomer of
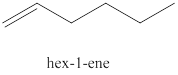
Figure 1
The second alkene isomer of
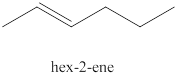
Figure 2
The third alkene isomer of
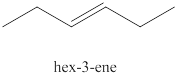
Figure 3
The fourth alkene isomer of
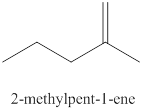
Figure 4
The fifth alkene isomer of
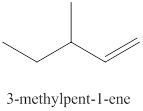
Figure 5
The sixth alkene isomer of
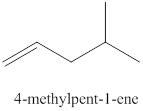
Figure 6
The seventh alkene isomer of
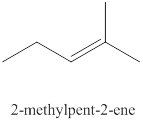
Figure 7
The eighth alkene isomer of
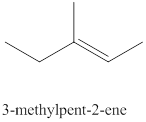
Figure 8
The ninth alkene isomer of
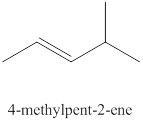
Figure 9
The tenth alkene isomer of
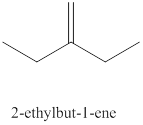
Figure 10
The eleventh alkene isomer of
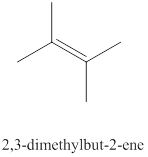
Figure 11
The twelfth alkene isomer of
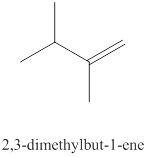
Figure 12
The thirteenth alkene isomer of
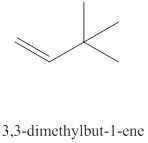
Figure 13
The structural formulas and the IUPAC names of the
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Write the molecular formula of each alkane.arrow_forwardWhich of the following alkenes can exist as cis-trans isomers? Draw structural formulas and name the cis and trans isomers. a.H2C=CH2CH3 b. c.arrow_forwardWhich of the following cycloalkanes could show geometric isomerism? For each that could, draw structural formulas, and name both the cis- and the trans- isomers. a. c. b. d.arrow_forward
- What is the difference in bonding and in the general molecular formula between an alkene and an alkane with the same number of carbon atoms?arrow_forwardWhy are different conformations of an alkane not considered structural isomers?arrow_forwardWhat is the difference in bonding and in general molecular formula between an alkene and a cycloalkane with the same number of carbon atoms?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





