
Concept explainers
Outline a two-dimensional unit cell for the pattern shown here. If the black squares are labeled A and the white squares are B, what is the simplest formula for a “compound” based on this pattern?
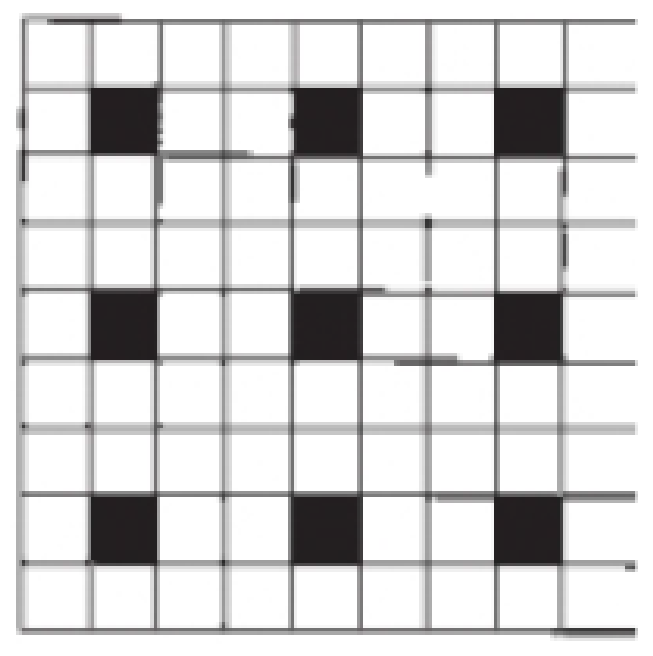
Interpretation:
The simplest formula of the compound has to be identified.
Answer to Problem 1PS
The simplest formula of the compound is
Explanation of Solution
According to the picture, we consider the unit pattern as combination of two molecules,
There are seventy two white squares and nine black squares in the two dimensional unit cell patterns.
The simplest formula of the compound was identified.
Want to see more full solutions like this?
Chapter 12 Solutions
OWLv2 6-Months Printed Access Card for Kotz/Treichel/Townsend's Chemistry & Chemical Reactivity, 9th, 9th Edition
Additional Science Textbook Solutions
Living By Chemistry: First Edition Textbook
Chemistry: Structure and Properties (2nd Edition)
Chemistry For Changing Times (14th Edition)
CHEMISTRY-TEXT
Elementary Principles of Chemical Processes, Binder Ready Version
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- Outline a two-dimensional unit cell for the pattern shown here. If the black squares are labeled A and the white squares are B, what is the simplest formula for a compound based on this pattern?arrow_forwardDescribe the crystal structure of Pt, which crystallizes with four equivalent metal atoms in a cubic unit cell.arrow_forwardLithium hydride (LiH) has the sodium chloride structure, and the length of the edge of the unit cell is 4.086 108 cm. Calculate the density of this solid.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





