College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter20: Electric Current, Resistance, And Ohm's Law
Section: Chapter Questions
Problem 47PE: Show that the units 1 A 2 = 1W, as implied by the equation P=I 2 R.
Related questions
Question
One mole of gas initially at a pressure of 2.00 atm and a volume of 0.300 L has an internal energy equal to 91.0 J. In its final state, the gas is at a pressure of 1.50 atm and a volume of 0.800 L, and its internal energy equals 182 J. For the paths IAF, IBF, and IF in Figure P12.30, calculate (a) the work done on the gas and (b) the net energy transferred to the gas by heat in the process.
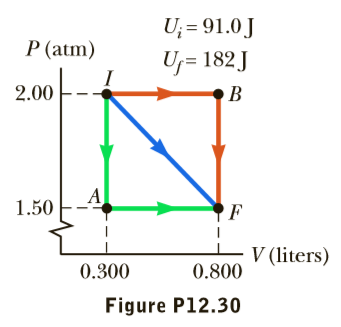
Transcribed Image Text:U; = 91.0 J
U;= 182J
P (atm)
2.00
1.50
V (liters)
0.300
0.800
Figure P12.30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning
