
Concept explainers
(a)
Interpretation:
The difference between ionic compound and molecular compound in scientific terms is to be stated.
Concept introduction:
An ionic bond is formed by the transfer of electrons, which is also known as electrovalent bond. The compound which are composed of molecules contain more than one type of atom are called molecular compounds.
Answer to Problem 41E
An ionic compound is formed by the transfer of electrons, while a molecular compounds are composed of molecules contain more than one type of atom.
Explanation of Solution
The compound which contains ionic bond between two or more atoms is known as ionic compound. Ionic bonds are formed between electropositive and electronegative elements, which have larger electronegativity difference. Sodium chloride is an example of ionic compound, which is shown below.
![]()
Figure 1
The compound which is composed of molecules containing more than one type of atom is called molecular compounds. Methane is an example of molecular compound, which is shown below.
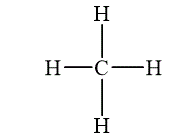
Figure 2
An ionic compound is formed by the transfer of electrons, while a molecular compounds are composed of molecules contain more than one type of atom.
(b)
Interpretation:
The difference between ionic bond and covalent bond in scientific terms is to be stated.
Concept introduction:
An ionic bond is formed by the transfer of electrons, which is also known as electrovalent bond. Ionic bonds are formed between electropositive and electronegative elements. A covalent bond is formed by the mutual sharing of electrons. The number of electrons contributed by each atom is known as covalence of the atom. The covalent bond is stronger than ionic bonds because of sharing of the electrons.
Answer to Problem 41E
An ionic bond is formed by the transfer of electrons, while a covalent bond is formed by the mutual sharing of electrons.
Explanation of Solution
An ionic bond is formed by the transfer of electrons, which is also known as electrovalent bond. Ionic bonds are formed between electropositive and electronegative elements. Sodium chloride is an example that includes ionic bonding, which is shown below.
![]()
Figure 1
A covalent bond is formed by the mutual sharing of the electrons. The number of electrons contributed by each atom is known as covalence of the atom. Hydrogen chloride is an example that describes covalent bonding, which is shown below.
![]()
Figure 3
An ionic bond is formed by the transfer of electrons, while a covalent bond is formed by the mutual sharing of electrons.
(c)
Interpretation:
The difference between lone pair and bonding pair in scientific terms is to be stated.
Concept introduction:
Lone pair of electrons is the type of electrons, which do not participate in the bond formation. Bonding pair of electrons are type of electrons, which participate in the bond formation and present between the bonded atoms.
Answer to Problem 41E
Lone pair of electrons are electrons, which do not take participate in the bond formation, while bonding pair of electron are electrons, which participate in the bond formation.
Explanation of Solution
Lone pair of electrons are electrons, which do not participate in the bond formation. Lone pair of electrons are localized on the atom. Bonding pair of electron are electrons, which participate in the bond formation and present between the bonded atoms. The example, which shows lone pair and bonding pair is shown below.
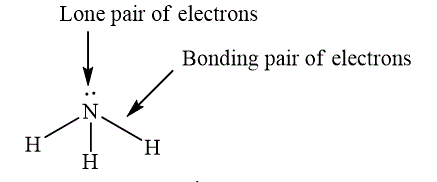
Figure 4
Loan pair of electrons are electrons, which do not participate in the bond formation, while bonding pair of electron are electrons, which participate in the bond formation.
(d)
Interpretation:
The difference between nonpolar bond and polar bond in scientific terms is to be stated.
Concept introduction:
Polar and non polar bonds are the type of covalent bond. A polar bond is formed between atoms of different elements, having some value of electronegativity difference. A non polar bond is formed between atoms of same elements.
Answer to Problem 41E
A polar bond is formed between atoms of different elements, while a non polar bond is formed between atoms of same elements.
Explanation of Solution
A polar bond is formed between atoms of different elements, having some value of electronegative difference. A non polar bond is formed between atoms of same elements.
The example of polar and non polar bond is shown below.
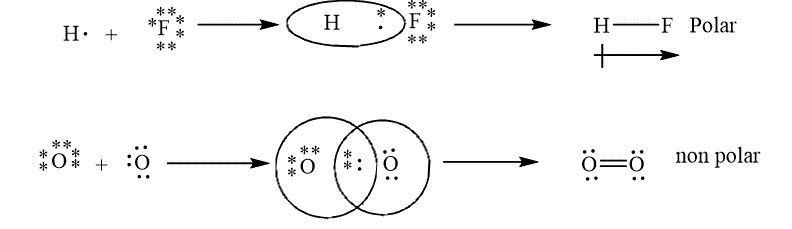
Figure 5
A polar bond is formed between atoms of different elements, while a non polar bond is formed between atoms of same elements.
(e)
Interpretation:
The difference among single, double, triple, and multiple bonds in scientific terms is to be stated.
Concept introduction:
If two atoms are bonded with the sharing of one pair of electron, such a bond is called as single bond. If two atoms are bonded by the sharing of two pairs of electrons, such a bond is called as double bond.
Answer to Problem 41E
Single bond is formed by the sharing of one pair of electron between two atoms. Double bond is formed by the sharing of two pair of electron between two atoms. Triple bond is formed by the sharing of three pair of electron between two atoms. Multiple bonds is the general term that includes double and triple bonds.
Explanation of Solution
If two atoms are bonded with the sharing of one pair of electron, such a bond is called as single bond. The example of a single bond is shown as below.
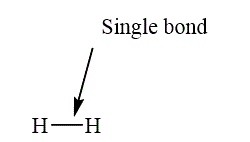
Figure 6
If two atoms are bonded by the sharing of two pairs of electrons, such a bond is called as double bond. The example of double bond is shown as below.
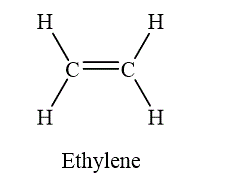
Figure 7
If two atoms are bonded by three pairs of electrons, such bond is called as triple bonds. The example of triple bond is shown as below.
![]()
Figure 8
Multiple bonds is a general term that includes double and triple bonds. The example of multiple bond is shown as below.
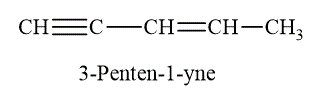
Figure 9
Single bond is formed by the sharing of one pair of electron between two atoms. Double bond is formed by the sharing of two pair of electron between two atoms. Triple bond is formed by the sharing of three pair of electron between two atoms. Multiple bonds is a general term that includes double and triple bonds.
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: An Active Learning Approach
- Write a formula for molecular compound. Xenon tetrafluoridearrow_forwardKevin is curious about the contrast dye and what ionic and covalent mean with regard to the element iodine. Which of the following statements regarding ionic and covalent bonds is/are true? Ionic bonds are formed by unequal sharing of electrons between two or more metal atoms. Covalent bonds form between metal and nonmetal atoms where one atom gains and the other loses electrons. Ionic bonds are formed by the equal sharing of electrons between two metal atoms. Ionic bonds are formed by the loss and gain of electrons between metal and nonmetal atoms. As the cardiologist explained, an iodine-containing dye used for angiograms is either an ionic compound or a covalent compound. Describe the difference between an ionic compound containing iodine and a covalent compound containing iodine.arrow_forwardWhich of the following does not describe an ionic bond? A. Between a cation and an anion B. Dissolves into ions in solution C. An unequal exchange of electrons D. An equal sharing of electronsarrow_forward
- Is it correct to say that a covalent bond is the electrostatic attraction between negative shared electrons and the positive nuclei of the two adjacent atomsarrow_forwardThe number of electrons that a double covalent bond will share is ________________.arrow_forwardplease answer correctly: What type of bond is in an ionic compound?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




