
Concept explainers
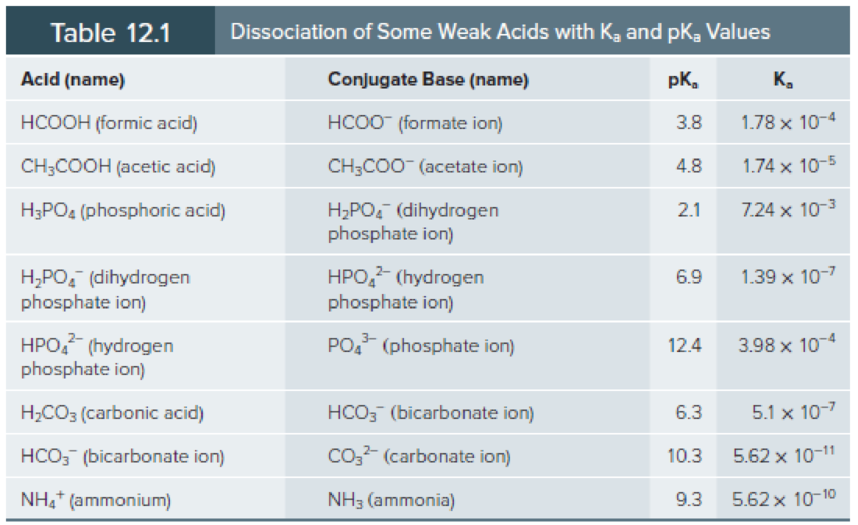
Use the Henderson-Hasselbalch equation and Table 12.1 to calculate the pH of the following solutions:
- a. 0.05 M formic acid and 0.1 M sodium formate.
- b. 0.2 M ammonium chloride and 0.1 M aqueous ammonia.
- c. 0.1 M acetic acid and 0.1 M sodium acetate.
(a)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
(b)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
(b)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry In Context
- The grid has six lettered boxes, each of which contains an item that may be used to answer the questions that follow. Items may be used more than once and there may be more than one correct item in response to a question. Place the letter(s) of the correct selection(s) on the appropriate line. Could dissolve Zn(OH)2 _____________ Could be used to prepare a buffer from __________ Halfway to the equivalence point in the titration of a weak, monoprotic acid with strong base ________ SO2-related atmospheric phenomenon __________ Species formed by a Lewis acid-base reaction ________ General condition required for precipitation to occur ________ [conj. base] = [conj. acid] in a buffer ___________arrow_forwardIn this activity, you will use the virtual lab to determine the concentration of a strong monoprotic acid. To do this, you can perform a titration using NaOH and phenolphthalein found in the virtual lab. (Note: The concentration of the acid is between 0.025M and 2.5M so you will need to dilute the NaOH solution so that the volume to reach the endpoint is between 10 and 50 mL). 1. Volume (mL) of the unknown acid solution with the indicator in the flask: ________ (Write your answer in 2 decimal places without the unit). 2. Volume (mL) of the base used to complete the titration:____________ (Write your answer in 2 decimal places without the unit). 3. Concentration (Molarity) of the unknown acid:_________ (Write your answer in 2 decimal places without the unit).arrow_forward-The solubility of Ag3PO4 is measured and found to be 1.99×10-3 g/L. Use this information to calculate a Ksp value for silver phosphate.Ksp = -The solubility of Zn(CN)2 is measured and found to be 1.45×10-2 g/L. Use this information to calculate a Ksp value for zinc cyanide. Ksp = Please help. Thank you.arrow_forward
- The solubility of manganese(II) hydroxide (Mn(OH)2) is 2.2 × 10-5 M. What is the Ksp of MnOH)2? (aq., 20 oC). A) 1.1 × 10-14B) 4.8 × 10-10C) 2.1 × 10-14D) 4.3 × 10-14 E) 2.2 × 10-5F) 3.1 × 10-15 G) 7.8 × 10-6arrow_forwardYou have been asked to make a guess as to the type of alkalinity present for the water samples listed below (hydroxide, carbonate, and/or bicarbonate) in each of the samples: (Use all the assumptions that we used in lab) Species present total ml to titrate all of the alkalinity due to the carbonate in the original solution A: Vp = 0 ml; Vmo = 0 ml B: Vp = 10 ml; Vmo = 10 ml C: Vp = 15 ml; Vmo = 5 ml D: Vp = 5 ml, Vmo = 10 mlarrow_forwardMake a scheme with this procedure. Write the result. Procedure 1. Place 20 drops of each of the following aqueous solutions to separate centrifuge tubes: 0.1M Cr (NO3)3, 0.1M Al (NO3)3, 0.1M Co (NO3)2, 0.1M Zn (NO3)2, 0.1M Mn (OH)2, 0.1M Ni (NO3)2, 0.1 M Fe (NO3)3. Make each solution basic by adding few drops of 6M NH4OH. Confirm using a litmus paper. 2. Add 5 drops of freshly prepared 6M (NH4)2S to each centrifuge tube. Place the samples in the centrifuge machine for 3 mins. After centrifuge record results. Decant the supernatant liquid of all the samples. 3. Add one drop of NH4OH in each centrifuge tubes. Add 20 drops of distilled water in each centrifuge tubes. Then add a few drops of 6M HCl in each solution. Place the samples in the water bath for 10 mins. After water bath, centrifuge the samples for 3 mins. 4. After centrifuge, add a few drops of 6M NH4Cl in each sample. Decant the supernatant liquid in each sample. 5. To the centrifuge containing Al+3 and Cr+3, slowly add…arrow_forward
- Complete the following: 1. Random error, also called _________arises from the effects of uncontrolled variables in the measurement. 2. The ____________,measures how closely the data are clustered about mean. 3. The chemical equilibrium in which all the reactants and products are in the same phase are called____________. 4. Reaction that go to completion and never proceed in the reverse direction are said to be_____________ 5. The equation for buffer is the__________equation. 6. A ___________solution is one that resists changes in pH when small quantities of an acid or an alkali added to it. 7. __________is the pH of the pure, neutral and polyproticarrow_forwardWhich are stronger or weaker? Product formation or reactor? HSO-44 (aq) + CO32- à SO42-(aq) HCO3-(aq) HPO42-(aq) + H2O(b) à H2PO4-(aq) + OH-(aq) NH4+ + OH-(aq) à NH3(aq) + H2O(b) Relative -strength of acids + base - conjugate acid/base pairs - direction of Rx <--- or --->arrow_forwardTime to purify biochemisfunase! To do this, you’ll need to make MORE buffers. One of the buffers that you need to make requires 150 mM Tris (pH 8), 250 mM sodium chloride (NaCl) and 2 mM dithiothreitol (DTT, a reducing agent that prevents the formation of disulfide bonds in proteins). Describe how you would make 1.5L of the buffer using your newly constructed 1.0 M Tris stock solution, a 500 mM stock solution of NaCl, and a 1.0 M stock solution of DTT. Show all calculations.arrow_forward
- Lactase is an enzyme that breaks down the sugar lactose which is commonly found in dairy products. Some people develop lactose intolerance as they grow older and the amount of lactase enzyme in their gut diminishes. Since the enzyme is commonly found in the gut of humans it can withstand a pH that is very acidic, ranging from a pH of 2-7. A student decided to do an experiment to determine how the pH of a solution affects lactase enzyme activity. Lactase works by breaking lactose into its two component sugars of glucose and galactose. To conduct the experiment the student prepared a solution of lactose and placed it into 5 different test tubes. A pH buffer solution was then added to each test tube with a pH of 2.0 in the first tube, 4.0 in the second, 6.0 in the third, 8.0 in the fourth, and a 10 pH in the fifth. Next, the enzyme lactase was added in equal amounts to each test tube and allowed to sit for ten minutes white the enzyme reacted with the lactose. At the end of the ten…arrow_forward0.15 gm of calcium carbonate is dissolved in 1 liter of distilled water. 20 ml of this standard hard water requires 25 ml of EDTA solution. 100 ml of unknown hard water requires 18 ml of EDTA solution. The same water sample after boiling requires 12 ml of EDTA solution. Calculate Temporary hardness of water sample in ppm.arrow_forwardAverage the two percentages and calculate the percentage difference. given : Concentration of acetic acid in the 100 mL sample of titration 1 = 3.238 % Concentration of acetic acid in the 100 mL sample of titration 2 = 3.099 % NaOH vs CH3COOH Burette solution is NaOH and the pipette solution is 5.0 mL of Vinegar Titration Initial burette reading Final burette reading Volume of NaOH consumed Average volume of NaOH Approximate 0.0 mL 20.1 mL 20.1 mL 20.3 mL Titration 1 0.0 mL 20.9 mL 20.9 mL Titration 2 0.0 mL 20.0 mL 20.0 mL To find the average volume Average volume = 20.1 mL + 20.9 mL + 20.0 mL320.1 mL + 20.9 mL + 20.0 mL3 = 20.3 mL Concentration of Vinegar = Volume of NaOH * Concentration of NaOHVolume of vinegarVolume of NaOH * Concentration of NaOHVolume of vinegar = 20.3 mL * 0.0647 M5.0 mL20.3 mL * 0.0647 M5.0 mL = 0.2627 M Concentration of NaOH = Volume of…arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

