
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 71SCQ
Phase diagrams for materials that have allotropes can be more complicated than those shown in the chapter. Use the phase diagram for carbon given here to answer the following questions.
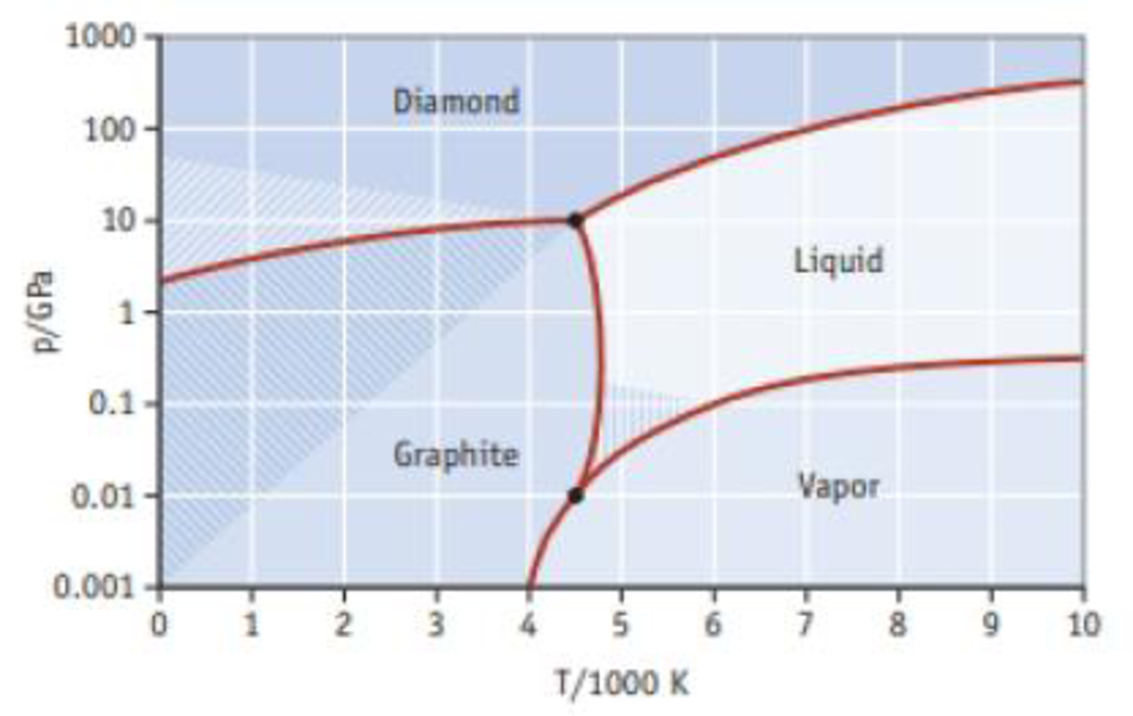
- (a) How many triple points are present and what phases are in equilibrium for each?
- (b) Is there a single point where all four phases are in equilibrium?
- (c) Which is more stable at high pressures, diamond or graphite?
- (d) Which is the stable phase of carbon at room temperature and 1 atmosphere pressure?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is represented by A on the phase diagram?
1.By understanding the structure of crystals molecularly, what is its relation to the strength of materials?
2. . Compare and contrast the two types of close-packing in solids
1. What phase change would occur iat a constant pressure of 0.25 atm the temperature was increased from 0C to 50C?
2.Trace the portion of the line representing melting/freezing with blue ink. Trace boiling/condensation with black ink and the portion representing sublimation in red ink?
3. At what point do all phases exist in equilibrium?
Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.6 - Prob. 1.1ACPCh. 12.6 - Describe the unit cell of lithium (see Figure).Ch. 12.6 - Prob. 1.3ACPCh. 12.6 - Prob. 1.4ACPCh. 12.6 - Prob. 2.1ACPCh. 12.6 - Prob. 2.2ACPCh. 12.6 - Prob. 2.3ACP
Ch. 12.6 - How many tin atoms are contained in the tetragonal...Ch. 12.6 - Prob. 3.2ACPCh. 12.6 - Prob. 3.3ACPCh. 12.6 - Prob. 3.4ACPCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - A portion of the crystalline lattice for potassium...Ch. 12 - The unit cell of silicon carbide, SiC, is...Ch. 12 - Prob. 5PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 14PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 17PSCh. 12 - Prob. 18PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - Prob. 25PSCh. 12 - Prob. 26PSCh. 12 - Prob. 27PSCh. 12 - Prob. 28PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 32PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 34PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 37PSCh. 12 - Prob. 38PSCh. 12 - Prob. 39PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 47GQCh. 12 - Prob. 48GQCh. 12 - Prob. 49GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Prob. 57GQCh. 12 - Prob. 58GQCh. 12 - Prob. 59GQCh. 12 - Prob. 60GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 67SCQCh. 12 - Prob. 68SCQCh. 12 - Prob. 69SCQCh. 12 - Phase diagrams for materials that have allotropes...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
Write a Lewis formula for each of the following organic molecules: C2H3Cl (vinyl chloride: starting material fo...
Organic Chemistry - Standalone book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which solid phase that is, which allotrope of carbon is more stable, graphite or diamond? You should consult some of the tables in the thermodynamics section of this text.. Both solid phases exist under normal conditions of pressure and temperature. Explain why this is so, given that one solid phase is more thermodynamically stable than the other. Do their unit cells provide any suggestion for their relative stabilities?arrow_forwardGiven the following data about xenon, normalboilingpoint=108Cnormalmeltingpoint=112Ctriplepoint=121Cat281mmHgcriticalpoint=16.6Cat58atm (a) Construct an approximate phase diagram for xenon. (b) Estimate the vapor pressure of xenon at -115C. (c) Is the density of solid Xe larger than that for liquid Xe?arrow_forwardWhat is the coordination number of Cs in CsCl? of Na in NaCl? of Zn2 in ZnS?arrow_forward
- What is the coordination number in the cesium chloride cubic structure?arrow_forwardList the different phase transitions that are possible and give examples of each.arrow_forwardSilane SiH4, phosphine (PH3), and hydrogen sulfide (H2S) melt at 185 C, 133 C, and 85 C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?arrow_forward
- The phase diagram for water over a relative narrow pressure and temperature range is given in Figure 9.19. A phase diagram over a considerably wider range of temperature and pressure (kbar) is given nearby. This phase diagram illustrates the polymorphism of ice, the existence of a solid in more than one form. In this case, Roman numerals are used to designate each polymorphic form. For example, Ice I, ordinary ice, is the form that exists under ordinary pressures. The other forms exist only at higher pressures, in some cases extremely high pressure such as Ice VII and Ice VIII. Using the phase diagram, give the approximate P and T conditions at the triple point for Ice III, Ice V, and liquid water. Determine the approximate temperature and pressure for the triple point for Ices VI, VII, and VIII. What is anomalously different about the fusion curves for Ice VI and Ice VII compared to that of Ice I? What phases exist at 8 kbar and 20 °C? At a constant temperature of −10 °C, start at 3 kbar and increase the pressure to 7 kbar. Identify all the phase changes that occur sequentially as these conditions change. Explain why there is no triple point for the combination of Ice VII, Ice VIII, and liquid water.arrow_forwardDescribe the major features of the phase diagram?arrow_forwardSulfur exhibits two solid phases, rhombic and monoclinic. Use the accompanying phase diagram for sulfur given below to answer the questions that follow. (A). How many triple points are in the phase diagram? (B). What phases are in equilibrium at the topmost triple point? (C). How many phase transitions are there when rhombic sulfur at 1 atm and 80°C is heated to 500°C at constant pressure?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Intermolecular Forces and Boiling Points; Author: Professor Dave Explains;https://www.youtube.com/watch?v=08kGgrqaZXA;License: Standard YouTube License, CC-BY