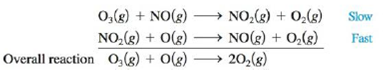
Problem 1RQ: Define reaction rate. Distinguish between the initial rate, average rate, and instantaneous rate of... Problem 2RQ: Distinguish between the differential rate law and the integrated rate law. Which of these is often... Problem 3RQ: One experimental procedure that can be used to determine the rate law of a reaction is the method of... Problem 4RQ: The initial rate for a reaction is equal to the slope of the tangent line at t 0 in a plot of [A]... Problem 5RQ: Consider the zero-, first-, and second-order integrated rate laws. If you have concentration versus... Problem 6RQ: Derive expressions for the half-life of zero-, first-, and second-order reactions using the... Problem 7RQ Problem 8RQ: What two requirements must be met to call a mechanism plausible? Why say a plausible mechanism... Problem 9RQ Problem 10RQ: Give the Arrhenius equation. Take the natural log of both sides and place this equation in the form... Problem 11RQ: Why does a catalyst increase the rate of a reaction? What is the difference between a homogeneous... Problem 1ALQ: Define stability from both a kinetic and thermodynamic perspective. Give examples to show the... Problem 2ALQ: Describe at least two experiments you could perform to determine a rate law. Problem 3ALQ: Make a graph of [A] versus time for zero-, first-, and second-order reactions. From these graphs,... Problem 4ALQ: How does temperature affect k, the rate constant? Explain. Problem 5ALQ: Consider the following statements: In general, the rate of a chemical reaction increases a bit at... Problem 6ALQ: For the reaction A+BC, explain at least two ways in which the rate law could be zero order in... Problem 7ALQ: A friend of yours states, A balanced equation tells us how chemicals interact. Therefore, we can... Problem 8ALQ: Provide a conceptual rationale for the differences in the half-lives of zero-, first-, and... Problem 9ALQ: The rate constant (k) depends on which of the following (there may be more than one answer)? a. the... Problem 10Q: Each of the statements given below is false. Explain why. a. The activation energy of a reaction... Problem 11Q: Define what is meant by unimolecular and bimolecular steps. Why are termolecular steps infrequently... Problem 12Q: The plot below shows the number of collisions with a particular energy for two different... Problem 13Q: For the reaction O2(g)+2NO(g)2NO2(g) the observed rate law is Rate=k[NO]2[O2] Which of the changes... Problem 14Q: The rate law for a reaction can be determined only from experiment and not from the balanced... Problem 15Q: Table 12.2 illustrates how the average rate of a reaction decreases with time. Why does the average... Problem 16Q: The type of rate law for a reaction, either the differential rate law or the integrated rate law, is... Problem 17Q: The initial rate of a reaction doubles as the concentration of one of the reactants is quadrupled.... Problem 18Q: Hydrogen reacts explosively with oxygen. However, a mixture of H2 and O2 can exist indefinitely at... Problem 19Q: The central idea of the collision model is that molecules must collide in order to react. Give two... Problem 20Q: Consider the following energy plots for a chemical reaction when answering the questions below. a.... Problem 21Q Problem 22Q: Would the slope of a ln(k) versus 1/T plot (with temperature in kelvin) for a catalyzed reaction be... Problem 23E: Consider the reaction 4PH3(g)P4(g)+6H2(g) If, in a certain experiment, over a specific time period,... Problem 24E: In the Haber process for the production of ammonia, N2(g)+3H2(g)2NH3(g) what is the relationship... Problem 25E: At 40C, H2O2 (aq) will decompose according to the following reaction: 2H2O2(aq)2H2O(l)+O2(g) The... Problem 26E: Consider the general reaction aA+bBcC and the following average rate data over some time period t:... Problem 27E: What are the units for each of the following if the concentrations are expressed in moles per liter... Problem 28E: The rate law for the reaction Cl2(g)+CHCl3(g)HCl(g)+CCl4(g) is Rate=k[Cl2]1/2[CHCl3] What are the... Problem 29E: The reaction 2NO(g)+Cl2(g)2NOCl(g) was studied at 10C. The following results were obtained where... Problem 30E: The reaction 2I-(aq)+S2O82-(aq)I2(aq)+2SO42-(aq) was studied at 25C. The following results were... Problem 31E: The decomposition of nitrosyl chloride was studied: 2NOCl(g) 2NO(g) + Cl2(g) The following data... Problem 32E: The following data were obtained for the gas-phase decomposition of dinitrogen pentoxide,... Problem 33E: The reaction I(aq)+OCl(aq)IO(aq)+Cl(aq) was studied, and the following data were obtained:... Problem 34E: The reaction 2NO(g)+O2(g)2NO2(g) was studied. and the following data were obtained where Rate=[O2]t... Problem 35E: The rote of the reaction between hemoglobin (Hb) and carbon monoxide (CO) was studied at 20C. The... Problem 36E: The following data were obtained for the reaction 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(l)... Problem 37E: The decomposition of hydrogen peroxide was studied, and the following data were obtained at a... Problem 38E: A certain reaction has the following general form: aAbB At a particular temperature and [A]0 = 2.00 ... Problem 39E: The rate of the reaction NO2(g)+CO(g)NO(g)+CO2(g) depends only on the concentration of nitrogen... Problem 40E: A certain reaction has the following general form: aAbB At a particular temperature and [A]0 = 2.80 ... Problem 41E: The decomposition of ethanol (C2H5OH) on an alumina (Al2O3) surface C2H5OH(g)C2H4(g)+H2O(g) was... Problem 42E: At 500 K in the presence of a copper surface, ethanol decomposes according to the equation... Problem 43E: The dimerization of butadiene 2C4H6(g)C8H12(g) was studied at 500. K, and the following data were... Problem 44E: The rate of the reaction O(g)+NO2(g)NO(g)+O2(g) was studied at a certain temperature. a. In one... Problem 45E: Experimental data for the reaction A2B+C have been plotted in the following three different ways... Problem 46E Problem 47E: The reaction AB+C is known to be zero order in A and to have a rate constant of 5.0 102 mol/L s at... Problem 48E: The decomposition of hydrogen iodide on finely divided gold at 150C is zero order with respect to... Problem 49E Problem 50E: A first-order reaction is 75.0% complete in 320. s. a. What are the first and second half-lives for... Problem 51E: The rate law for the decomposition of phosphine (PH3) is Rate=[PH3]t=k[PH3] It takes 120. s for 1.00... Problem 52E: DDT (molar mass = 354.49 g/mol) was a widely used insecticide that was banned from use in the United... Problem 53E: Consider the following initial rate data for the decomposition of compound AB to give A and B: [AB]o... Problem 54E: The rate law for the reaction 2NOBr(g)2NO(g)+Br2(g) at some temperature is Rate=[NOBr]t=k[NOBr]2 a.... Problem 55E Problem 56E: Theophylline is a pharmaceutical drug that is sometimes used to help with lung function. You observe... Problem 57E: You and a coworker have developed a molecule thathas shown potential as cobra antivenin (AV). This... Problem 58E: Consider the hypothetical reaction A+B+2C2D+3E where the rate law is Rate=[A]t=k[A][B]2 An... Problem 59E: Write the rate laws for the following elementary reactions. a. CH3NC(g) CH3CN(g) b. O3(g) + NO(g) ... Problem 60E: A possible mechanism for the decomposition of hydrogen peroxide is H2O22OHH2O2+OHH2O+HO2HO2+OHH2O+O2... Problem 61E: A proposed mechanism for a reaction is... Problem 62E: The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric... Problem 63AE: For the following reaction profile, indicate a. the positions of reactants and products. b. the... Problem 64AE: Draw a rough sketch of the energy profile for each of the following cases: a. E = + 10 kJ/mol, Ea =... Problem 65AE: The activation energy for the reaction NO2(g)+CO(g)NO(g)+CO2(g) is 125 kJ/mol, and E for the... Problem 66AE: The activation energy for some reaction X2(g)+Y2(g)2XY(g) is 167 kJ/mol, and E for the reaction is +... Problem 67AE: The rate constant for the gas-phase decomposition of N2O5, N2O52NO2+12O2 has the following... Problem 68AE: The reaction (CH3)3CBr+OH(CH3)3COH+Br in a certain solvent is first order with respect to (CH3)3CBr... Problem 69AE: The activation energy for the decomposition of HI(g) to H2(g) and I2(g) is 186 kJ/mol. The rate... Problem 70AE: A first-order reaction has rate constants of 4.6 102 s1 and 8.1 102 s1 at 0C and 20.C,... Problem 71AE: A certain reaction has an activation energy of 54.0 kJ/mol. As the temperature is increased from 22C... Problem 72AE Problem 73AE: Which of the following reactions would you expect to proceed at a faster rate at room temperature?... Problem 74AE Problem 75AE: One mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a... Problem 76AE: One of the concerns about the use of Freons is that they will migrate to the upper atmosphere, where... Problem 77AE: Assuming that the mechanism for the hydrogenation of C2H4 given in Section 11-7 is correct, would... Problem 78AE: The decomposition of NH3 to N2 and H2 was studied on two surfaces: Surface Ea (kJ/mol) W 163 Os 197... Problem 79AE: The decomposition of many substances on the surface of a heterogeneous catalyst shows the following... Problem 80AE Problem 81AE: A popular chemical demonstration is the magic genie procedure, in which hydrogen peroxide decomposes... Problem 82AE Problem 83AE: Consider the following representation of the reaction 2NO2(g) 2NO(g) + O2(g). Determine the time... Problem 84AE: The reaction H2SeO3(aq) + 6I-(aq) + 4H+(aq) Se(s) + 2I-3(aq) + 3H2O(l) was studied at 0C, and the... Problem 85AE: Consider two reaction vessels, one containing A and the other containing B, with equal... Problem 86AE: Sulfuryl chloride (SO2Cl2) decomposes to sulfur dioxide (SO2) and chlorine (Cl2) by reaction in the... Problem 87AE: For the reaction 2N2O5(g)4NO2(g)+O2(g) the following data were collected, where Rate=[N2O5]t Time... Problem 88AE: Experimental values for the temperature dependence of the rate constant for the gas-phase reaction... Problem 89AE: Cobra venom helps the snake secure food by binding to acetylcholine receptors on the diaphragm of a... Problem 90AE: Iodomethane (CH3I) is a commonly used reagent in organic chemistry. When used properly, this reagent... Problem 91AE: Experiments during a recent summer on a number of fireflies (small beetles, Lampyridaes photinus)... Problem 92AE: The activation energy of a certain uncatalyzed biochemical reaction is 50.0 kJ/mol. In the presence... Problem 93AE: Consider the reaction 3A+B+CD+E where the rate law is defined as [A]t=k[A]2[B][C] An experiment is... Problem 94CWP: The thiosulfate ion (S2O32) is oxidized by iodine as follows: 2S2O32(aq)+I2(aq)S4O62(aq)+2I(aq) In a... Problem 95CWP: The reaction A(aq)+B(aq)products(aq) was studied, and the following data were obtained: [Al]0... Problem 96CWP: A certain substance, initially present at 0.0800 M, decomposes by zero-order kinetics with a rate... Problem 97CWP: A reaction of the form aAProducts gives a plot of ln[A] versus time (in seconds), which is a... Problem 98CWP: A certain reaction has the form aAProducts At a particular temperature, concentration versus time... Problem 99CWP Problem 100CWP: Consider the hypothetical reaction A2(g) + B2(g) 2AB(g), where the rate law is: [A2]t=k[A2][B2] The... Problem 101CWP: Experiments have shown that the average frequency of chirping by a snowy tree cricket (Oecanthus... Problem 102CP: Consider a reaction of the type aA products, in which the rate law is found to he rate = k[A]3... Problem 103CP: A study was made of the effect of the hydroxide concentration on the rate of the reaction... Problem 104CP: Two isomers (A and B) of a given compound dimerize as follows: 2Ak1A22Bk2B2 Both processes are known... Problem 105CP: The reaction NO(g)+O3NO2(g)+O2(g) was studied by performing two experiments. In the first experiment... Problem 106CP: Most reactions occur by a series of steps. The energy profile for a certain reaction that proceeds... Problem 107CP: You are studying the kinetics of the reaction H2(g) + F2(g) 2HF(g) and you wish to determine a... Problem 108CP: The decomposition of NO2(g) occurs by the following bimolecular elementary reaction:... Problem 109CP: The following data were collected in two studies of the reaction 2A+BC+D Time (s) Experiment 1 [A]... Problem 110CP: Consider the following hypothetical data collected in two studies of the reaction 2A+2BC+2D Time(s)... Problem 111CP: Consider the hypothetical reaction A+B+2C2D+3E In a study of this reaction three experiments were... Problem 112CP: Hydrogen peroxide and the iodide ion react in acidic solution as follows:... Problem 113IP: Sulfuryl chloride undergoes first-order decomposition at 320.C with a half-life of 8.75 h.... Problem 114IP: Upon dissolving InCl(s) in HCl, In+(aq) undergoes a disproportionation reaction according to the... Problem 115IP: The decomposition of iodoethane in the gas phase proceeds according to the following equation:... Problem 116MP: Consider the following reaction: CH3X+YCH3Y+X At 25C, the following two experiments were run,... format_list_bulleted


 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax




