
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 7PS
Cuprite is a semiconductor. Oxide ions are at the cube comers and in the cube center. Copper ions are wholly within the unit cell.
- (a) What is the formula of cuprite?
- (b) What is the oxidation number of copper?
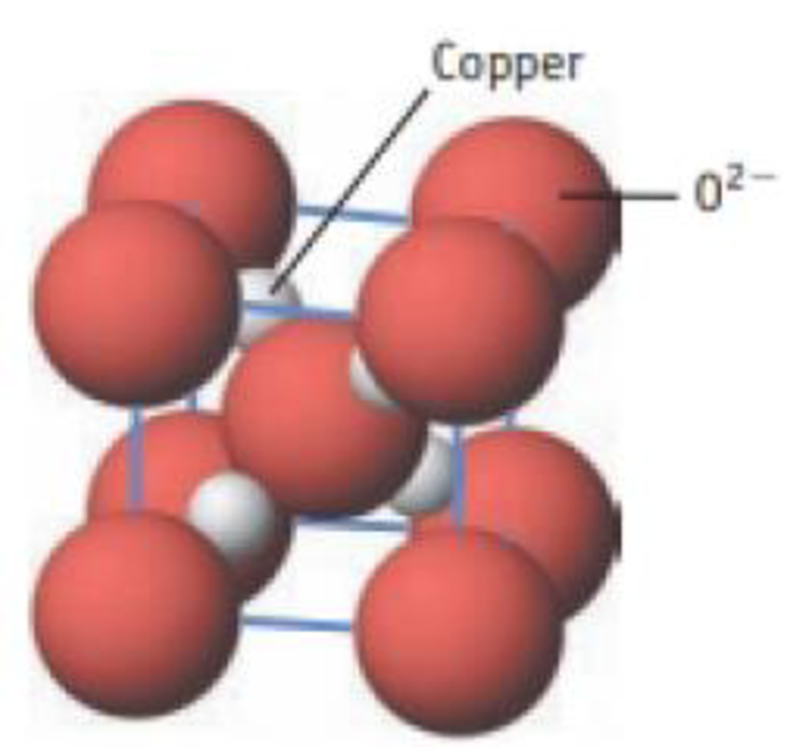
Unit cell for cuprite
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 12 Solutions
Chemistry & Chemical Reactivity
Ch. 12.1 - (a) Determining an Atom Radius from Lattice...Ch. 12.2 - If an ionic solid has an fcc lattice of anions (X)...Ch. 12.2 - Potassium chloride has the same unit cell as NaCl....Ch. 12.6 - Prob. 1.1ACPCh. 12.6 - Describe the unit cell of lithium (see Figure).Ch. 12.6 - Prob. 1.3ACPCh. 12.6 - Prob. 1.4ACPCh. 12.6 - Prob. 2.1ACPCh. 12.6 - Prob. 2.2ACPCh. 12.6 - Prob. 2.3ACP
Ch. 12.6 - How many tin atoms are contained in the tetragonal...Ch. 12.6 - Prob. 3.2ACPCh. 12.6 - Prob. 3.3ACPCh. 12.6 - Prob. 3.4ACPCh. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - Outline a two-dimensional unit cell for the...Ch. 12 - A portion of the crystalline lattice for potassium...Ch. 12 - The unit cell of silicon carbide, SiC, is...Ch. 12 - Prob. 5PSCh. 12 - Rutile, TiO2, crystallizes in a structure...Ch. 12 - Cuprite is a semiconductor. Oxide ions are at the...Ch. 12 - The mineral fluorite, which is composed of calcium...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - The density of copper metal is 8.95 g/cm3. If the...Ch. 12 - Potassium iodide has a face-centered cubic unit...Ch. 12 - A unit cell of cesium chloride is illustrated in...Ch. 12 - Predict the trend in lattice energy, from least...Ch. 12 - Prob. 14PSCh. 12 - To melt an ionic solid, energy must be supplied to...Ch. 12 - Which compound in each of the following pairs...Ch. 12 - Prob. 17PSCh. 12 - Prob. 18PSCh. 12 - Considering only the molecular orbitals formed by...Ch. 12 - Prob. 20PSCh. 12 - Prob. 21PSCh. 12 - Prob. 22PSCh. 12 - Prob. 23PSCh. 12 - Prob. 24PSCh. 12 - Prob. 25PSCh. 12 - Prob. 26PSCh. 12 - Prob. 27PSCh. 12 - Prob. 28PSCh. 12 - A diamond unit cell is shown here. Unit cell of...Ch. 12 - The structure of graphite is given in Figure...Ch. 12 - We have identified six types of solids (metallic,...Ch. 12 - Prob. 32PSCh. 12 - Classify each of the following materials as...Ch. 12 - Prob. 34PSCh. 12 - Benzene, C6H6, is an organic liquid that freezes...Ch. 12 - The specific heat capacity of silver is 0.235 J/g ...Ch. 12 - Prob. 37PSCh. 12 - Prob. 38PSCh. 12 - Prob. 39PSCh. 12 - If your air conditioner is more than several years...Ch. 12 - Sketch a phase diagram for O2 from the following...Ch. 12 - Tungsten crystallizes in the unit cell shown here....Ch. 12 - Silver crystallizes in a face-centered cubic unit...Ch. 12 - The unit cell shown here is for calcium carbide....Ch. 12 - The very dense metal iridium has a face-centered...Ch. 12 - Vanadium metal has a density of 6.11 g/cm3....Ch. 12 - Prob. 47GQCh. 12 - Prob. 48GQCh. 12 - Prob. 49GQCh. 12 - Consider the three types of cubic units cells. (a)...Ch. 12 - The solid-state structure of silicon is shown...Ch. 12 - The solid-state structure of silicon carbide is...Ch. 12 - Spinels are solids with the general formula AB2O4...Ch. 12 - Using the thermochemical data below and an...Ch. 12 - Prob. 55GQCh. 12 - Prob. 56GQCh. 12 - Prob. 57GQCh. 12 - Prob. 58GQCh. 12 - Prob. 59GQCh. 12 - Prob. 60GQCh. 12 - Like ZnS, lead(II) sulfide, PbS (commonly called...Ch. 12 - CaTiO3, a perovskite, has the structure below. (a)...Ch. 12 - Potassium bromide has the same lattice structure...Ch. 12 - Calculate the lattice energy of CaCl2 using a...Ch. 12 - Why is it not possible for a salt with the formula...Ch. 12 - Prob. 67SCQCh. 12 - Prob. 68SCQCh. 12 - Prob. 69SCQCh. 12 - Phase diagrams for materials that have allotropes...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The unit cell of silicon carbide, SiC, is illustrated below. (a) In what type of unit cell are the (dark gray) C atoms arranged? (b) If one edge of the silicon carbide unit cell is 436.0 pm, what is the calculated density of this compound? A portion of the solid-state structure of silicon carbide.arrow_forwardRutile, TiO2, crystallizes in a structure characteristic of many other ionic compounds How many formula units of TiO2 are in the unit cell illustrated here? (The oxide ions marked by an x are wholly within the cell; the others are in the cell faces.) Unit cell for rufflearrow_forwardWhat is the coordination number of Cs in CsCl? of Na in NaCl? of Zn2 in ZnS?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY