
Classify the carbon atoms in each compound as 1°, 2°, 3°, or 4°.
a.
b.
(a)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
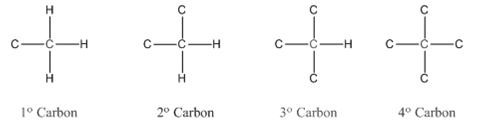
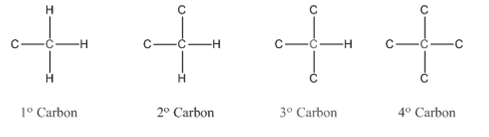
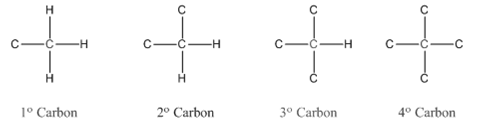
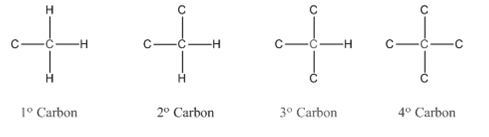
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
Explanation of Solution
The given compound is
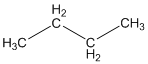
According to the above structure of compound, the terminal carbons present on both sides of the chain is classified as primary carbon as they are linked with one other carbon atom whereas carbon atoms which are present in between the terminal carbonsof the chain are linked with two other carbon atoms thus, classified as secondary carbon.
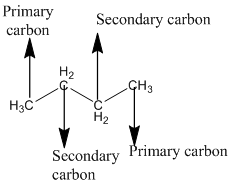
Thus, two primary and two secondary carbons are present in the structure.
(b)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
Explanation of Solution
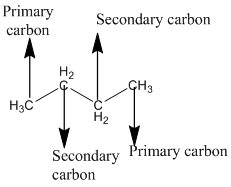
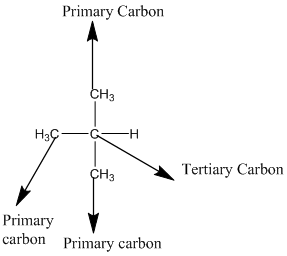
The given compound is
According to the above structure of compound, the terminal carbons (3 carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas carbon atom which is present in the middle linked with three other carbon atoms thus, classified as tertiary carbon.
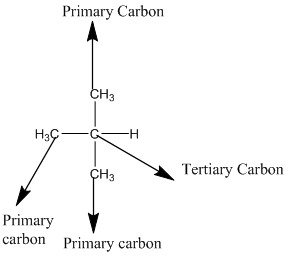
Thus, three primary carbons and onetertiary carbon atom are present in the structure.
(c)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
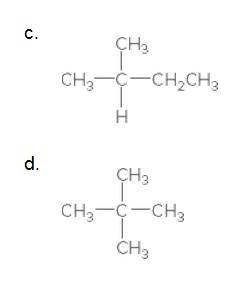
Answer to Problem 12.3PP
Explanation of Solution
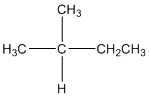
The given compound is
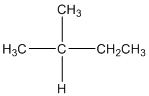
According to the above structure of compound, the terminal carbons (3 carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas the carbon which is linked with two other carbon atoms is classified as secondary carbon and the carbon which is linked with three other carbon atoms is classified as tertiary carbon.
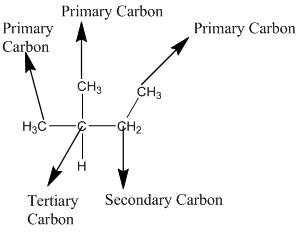
Thus, three primary carbons, one secondary carbon atom and one tertiary carbon atom are present in the structure.
(d)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
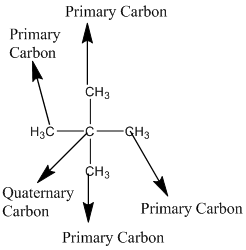
Explanation of Solution
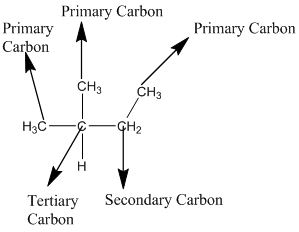
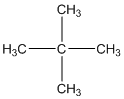
The given compound is
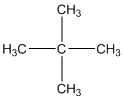
According to the above structure of compound, the terminal carbons (four carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas the carbon which is linked with four other carbon atoms is classified as quaternary carbon.
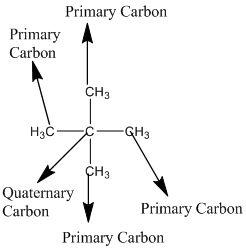
Thus, four primary carbons and one quaternary carbon atom are present in the structure.
Want to see more full solutions like this?
Chapter 12 Solutions
General, Organic, and Biological Chemistry - 4th edition
- How many moles of bonds between which pairs of atoms are broken during the combustion of 3 moles of methane (CH4) gas? Treat a double bond or a triple bond as one bonding interaction (i.e., 1 mole of triple bonds equals 1 mole of bonds). Choose one or more: A. 12 moles O–H bonds B. 6 moles of C–O bonds C. 3 moles of C–O bonds D. 6 moles of O–H bonds E. 3 moles of C–H bonds F. 6 moles of O–O bonds G. 12 moles of C–H bondsarrow_forwardN2(g) + 3H2(g) → 2NH3(g)ΔH = -92.2 kJ H2(g) + Cl2(g) → 2HCl(g)ΔH = -184.7 kJ N2(g) + 4H2(g) + Cl2(g) → 2NH4Cl(s)ΔH = -628.9 kJ Find the ΔH of the following reaction: NH3(g) + HCl(g) → NH4Cl(s)arrow_forwardA) Kb=[NH(+4)] B) Kb=[NH(+4)]/[OH(-)][NH3] C) Kb=[OH(-)]/[NH3] D) Kb=[NH(+4)]/[NH3]arrow_forward
- Calculate the overall value of ∆H° for this reaction. CH3¬CH3 + Cl2 ¡hv CH3¬CH2Cl + HClarrow_forward2 Q: Consider the following compound: CH3-CH2-CO-CH3 a. suggest a method for increasing the number of carbons by one: b. suggest a method for decreasing the number of carbons by one:arrow_forwardHOCI HF HCN H₂SO4 HOBr 2.29 x 10-8 O 2.30 K₂= 3.510-8 O 1.17 x 108 O 1.45 x 10-7 K₂ = 7.2 10-4 K₂ = 4.010-10 K₁ = very large K₂ = 1.2 10-2 Ka = 2.5210-⁹ (COOH)2 CH3COOH C6H5NH2 NH3 K₁ = 5.9 x 10-² K₂ = 6.4 x 10-5 K₂ = 1.8 x 10-5 Refer to Equilibrium Constants. What is the [H3O+] of a solution that is 0.0100 M in HOCland 0.0300 M in NaOCI? Kb = 4.2 x 10-10 Kb = 1.8 x 10-5arrow_forward
- Break compound A into several small (less or equals to 6 carbon atom) molecules. You need to explain the reasons why you choose to break those bonds.arrow_forwardthe context is "Cosmone is a molecule used by fragrance manufacturers to provide a rich and elegant musky essence to many perfumes. Cosmone has the molecular formula C15H26O." I need help on part (e) and (f).arrow_forwardWhat do we represent when we write:CH3 CO2 H(aq) + H2 O(l) ⇌ H3 O+(aq) + CH3 CO2−(aq)?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
