
You work in a developing nation for a large chemical company. Your division works on refrigerants and foam-blowing agents. You have need to correlate a set of data for the trifluoromethane (1) + trifluorochloromethane (2) system at 31.9 F. You know the following about your system:
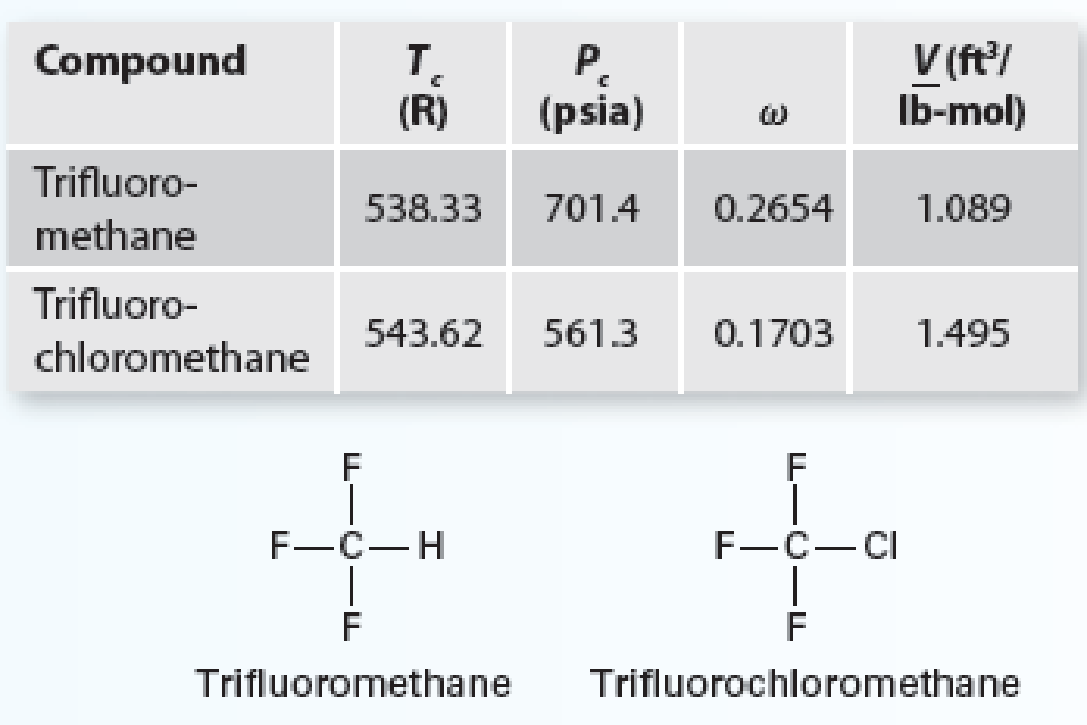
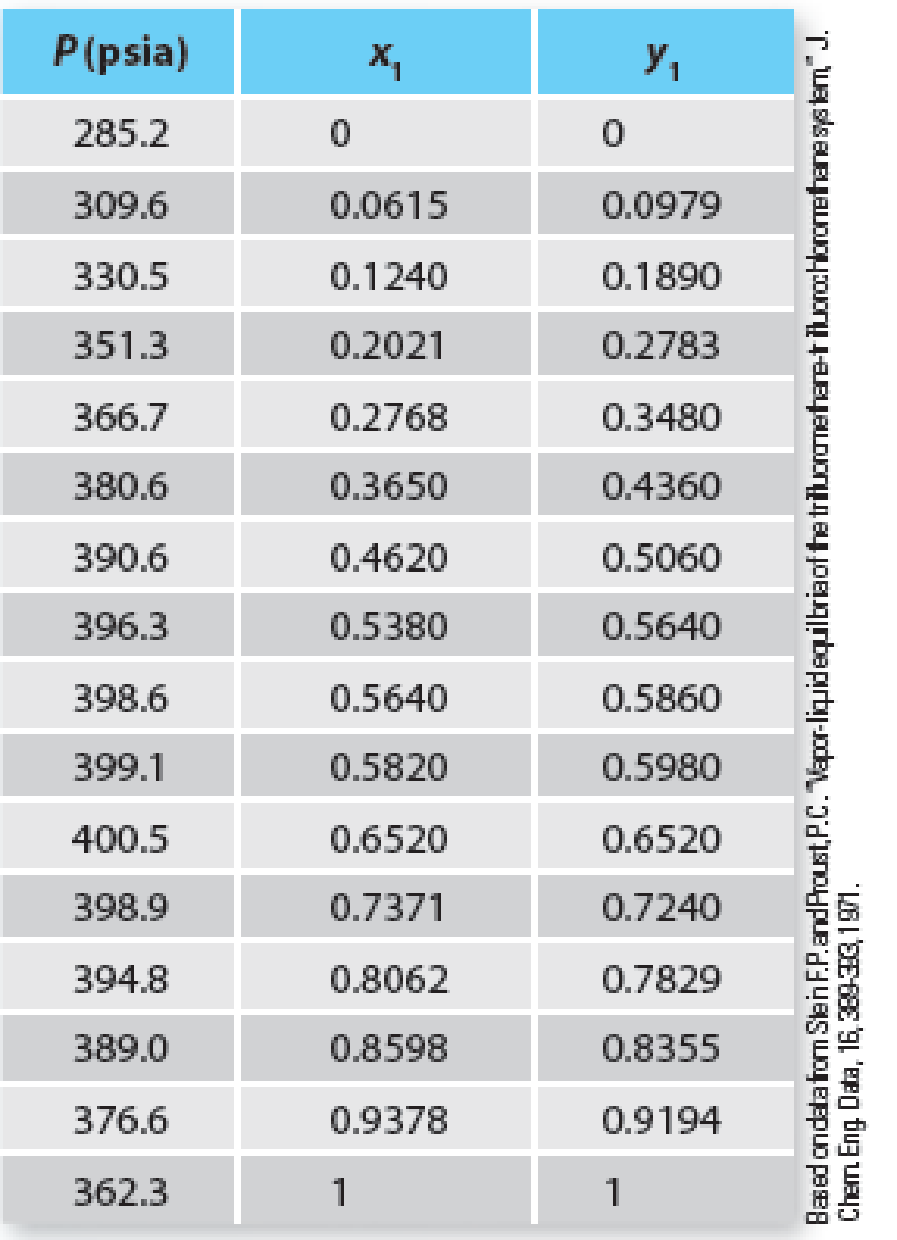
Using a gamma-phi modeling approach, calculate the Pxy diagram for the system using the Wilson equation and the virial equation. Compare the predicted values with the experimental data provided (on the same plot) in Table P12-19. Also, please provide two additional modeling approaches to the plot.
- 1. Model the system using modified Raoult’s Law (ideal gas for the vapor). This includes calculating the activity coefficients assuming an ideal gas for the vapor (as in Chapter 11).
- 2. Model the system using Raoult’s Law.
Table P12-19 Vapor-liquid equilibrium of trifluoromethane (1) + trifluorochloromethane (2) at 31.9°F.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Fundamentals of Chemical Engineering Thermodynamics (MindTap Course List)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





