
Concept explainers
a)
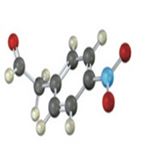
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the
Answer to Problem 12VC
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
Explanation of Solution
a) In the molecule C-H stretch shows an absorption at 3100 cm-1 , C=C
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
b)
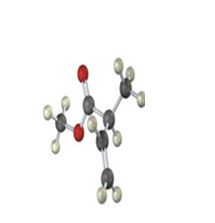
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
Explanation of Solution
In the molecule
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
c)
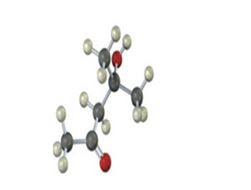
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Explanation of Solution
In the molecule alkane C-H stretch shows an absorption at 2850-2960 cm-1 , O-H stretching at 3500 cm-1. The carbonyl C=O shows an absorption at 1715 cm-1.
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry
- Which of these compounds matches to the IR spectrum?arrow_forwardFor each compound with formula C4H8O2, provide a brief reason why it could or could not match the IR spectrum.arrow_forwardCircle the solvents from the following list that can be used with 1H NMR spectroscopy that do not interfere with the spectrum. carbon tetrachloride chloroform benzene-d6 hexachloroacetone acetonitrile acetone methylene chloride D2O DMF (dimethylformamide)arrow_forward
- Label all major absorbance bands in the following IR spectrum specifying the absorptions associated with the C=O, C-O, and C-H bondsarrow_forwardIdentify the functional group of the molecule in the following IR spectrum.arrow_forwardBased on this portion of an IR spectra is there a ketone that is present?arrow_forward
- The ___________ functional group will have a sharp, dagger-like absorbance at 2220-2250 cm-1 on its IR spectrum (and an odd-numbered molecular mass (M+) on its mass spectrum). alkyne nitrile aldehyde alkene carbolxylic acidarrow_forwardHi, please help me to interpret this IR spectrum of Aspirin (acetylsalicylic acid)arrow_forwardWhich of the two IR spectra shown below refers to a carboxylic acid? Explain your answer with IR frequency values.arrow_forward
