
Concept explainers
The equilibrium constant for the conjugate acid-base pair
(a) calculate the absorbance at 430 nmand 600 nm for the following indicator concentrations:
3.00 × 10-4M,2.00 × 10-4M, 1.00 × 10-4M, 0.500 × 10-4 M, and 0.250 × 10-4M.
(b) plot absorbance as a function of indicator concentration.

(a)
Interpretation:
Absorbance values of the conjugate acid-base pair solutions of different concentrations at
Concept introduction:
The absorbance can be calculated using the following formula:
For the given conjugate acid-base pair, equilibrium can be written as,
Answer to Problem 13.11QAP
Explanation of Solution
Let’s use the equilibrium equation to find the concentrations of HIn and In-(denoted as [HIn] and [In-]) at a situation in which the total concentration (cHIn) is
Now, the absorbance values of this solution at 430 and 600 nm can be calculated as follows,
The cell length (b) is not given in the question, therefore taken as 1.00cm.
Similarly, other absorbance values can be calculated for solutions with different concentrations.
(b)
Interpretation:
Absorbance as a function of indicator concentration should be plotted.
Explanation of Solution
The data obtained is as follows:
From the data, the graph between absorbance and concentration can be plotted as follows:
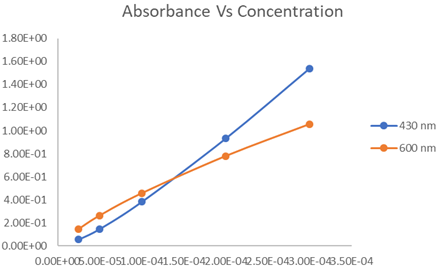
Here, blue curve indicates the plot at 430 nm and orange curve indicates the same at 600 nm.
Want to see more full solutions like this?
Chapter 13 Solutions
Principles of Instrumental Analysis
- Write equilibrium showing the carbonate anion (CO32-) acting as a Brønsted base in aqueous solution. Identify the conjugate base-acid (or acid-base) pairs and write the appropriate base dissociation constant (Kb) expression. Look up Kb value and compare to Kb of carbonate as 2.1 x 10-4. Did your value fall in the same range?arrow_forwardConsider that benzoic acid is a weak acid which has a Ka of 6.3 x 10-5 . C6H5COOH → C6H5COO- + H+ Calculate the [H+ ] ion concentration of a 0.04 M benzoic acid solution. Use your findings from above, determine the pH of the solution.arrow_forwardCalculate pH and pOH of the: 0.5M aniline (C6H5NH2) solution (Kb= 3.8 x 10-10)arrow_forward
- 1b) Suppose you decreased the pH of the biotin solution from 7.0 to 3.0 - what would happen to the ionizable group on a molecule of biotin as the pH shifted from 7.0 to 3.0? Briefly explain why you would expect that to happenarrow_forwardCalculate the pH (aq., 25 oC) of a 0.100 M triethylamine ((C2H5)3N) solution whose Kb = 1.02 x 10–3.arrow_forwardCalculate the pH of 0.1mol dm-3 potassium hydroxide (KOH(aq))arrow_forward
- What volume of 4.00 M HCL (aq) must be added to 10.0 mL of 2.00 M NH3 (aq) to obtain the solution buffered at pHbuffer= 8.00 at room temperature, if the base ionization constant of ammonia is Kb= 1.80 x 10-5 at this temperature?arrow_forwardCalculate the pH of the following acid solutions at 25 °C: (a) 1.0 x 10-4 M H3BO3(aq) (boric acid acts as a monoprotic acid), (b) 0.015 M H3PO4 (aq), (c) 0.10 M H2SO3(aq). Values for the successive acidity constants for each of these acids are given in the below table.arrow_forwardGive the pH of 0.005 M C6H5O- (aq) at 298 K of the Ka for C6H5OH at the given T is 1.0 x 10-10arrow_forward
- Calculate the pH of the following solutions:1) 0.0624 molar piperazine 2)A 0.0850 molar solution of the intermediate (HA) form of threonine. 3)A 0.0250 molar solution of potassium carbonate. 4. Calculate the pH of the following solutions:a. A solution prepared by combining 25.00 mL of 0.0486 molar sodium cyanide with 15.00 mL of 0.0876 molar HCl. b. A solution prepared by combining 35.00 mL of 0.0728 molar hypochlorous acid with 12.00 mL of 0.0967 molar NaOH. 5. Consider the titration of 30.00 mL of 0.0500 molar ammonia with 0.0250 molar HCl.a. What is the pH of this solution at the equivalence point? b. Using the table of indicators in the text, choose the ideal indicator to detect this endpoint. Explain your choice.arrow_forwardCalculate the pH, pOH, and fraction of solute protonated or deprotonated in the following aqueous solutions: (a) 0.150 M CH3CH(OH)COOH(aq) (lactic acid), (b) 2.4 x 10-4 M CH3CH(OH)COOH(aq), (c) 0.25 M C6H5SO3H(aq) (benzenesulfonic acid). The appropriate values for pKa or pKb are given inthe table.arrow_forwardWhat is the pH of rainwater at 25°C in which atmospheric CO2 has dissolved, producing an initial [H2CO3] of 1.28×10-5 M ? Take into account the autoionization of water.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

