
(a)
Interpretation:
The name of the given compound should be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other
The name of the given compound is 4-Chloronitrobenzene.
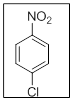
In the given structure there are two substituents. Those are nitro and chloro. Out of them, nitro gets priority over chloro and thus the name is 4-chloronitrobenzene as the chloro is at
Explanation of Solution

In the given structure there are two substituents. Those are nitro and chloro. Out of them, nitro gets priority over chloro and thus the name is 4-chloronitrobenzene as the chloro is at
(b)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 2-bromotoluene.
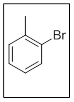
In the given structure there are two substituents. Those are methyl and bromo. Out of them methyl gets priority over bromo and thus the name is 2-bromotoluene as the bromo is at
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between methyl and bromo, bromo is the substituent as all the
Explanation of Solution

In the given structure there are two substituents. Those are methyl and bromo. Out of them methyl gets priority over bromo and thus the name is 2-bromotoluene as the bromo is at
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between methyl and bromo, bromo is the substituent as all the haloalkanes are named as substituted alkane.
(c)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 1-chloro-3-phenylpropane.

In the given structure there are two substituents. Those are chloro and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between chloro and phenyl chloro gets priority over phenyl due to alphabetical order and both of chloro and phenyl are the substituents. All the haloalkanes are named as substituted alkanes.
Explanation of Solution

In the given structure there are two substituents. Those are chloro and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between chloro and phenyl chloro gets priority over phenyl due to alphabetical order and both of chloro and phenyl are the substituents. All the haloalkanes are named as substituted alkanes.
(d)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 2-bromo-2-phenylbutane.
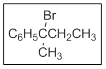
In the given structure there are two substituents. Those are bromo and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between bromo and phenyl bromo gets priority over phenyl due to alphabetical order and both of bromo and phenyl are the substituents. All the haloalkanes are named as substituted alkanes.
Explanation of Solution
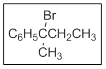
In the given structure there are two substituents. Those are bromo and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between bromo and phenyl bromo gets priority over phenyl due to alphabetical order and both of bromo and phenyl are the substituents. All the haloalkanes are named as substituted alkanes.
(e)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 2-nitroaniline.
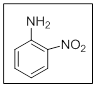
In the given structure there are two substituents. Those are nitro and amino at position
Explanation of Solution

In the given structure there are two substituents. Those are nitro and amino at position
(f)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 2-phenylphenol.
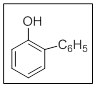
In the given structure there are two substituents. Those are hydroxyl and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group.
Phenyl is an aryl substituent where as phenolic hydroxyl group is a functional group. Thus hydroxybenzene is the functional group with phenyl as substituent. Thus the name is 2-phenylphenol.
Explanation of Solution

In the given structure there are two substituents. Those are hydroxyl and phenyl at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group.
Phenyl is an aryl substituent where as phenolic hydroxyl group is a functional group. Thus hydroxybenzene is the functional group with phenyl as substituent. Thus the name is 2-phenylphenol.
(g)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is trans stilbene.
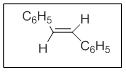
In the given structure there are two substituents. Those are phenyl at position
Thus the name is trans stilbene.
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group.
Explanation of Solution

In the given structure there are two substituents. Those are phenyl at position
Thus the name is trans stilbene.
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group.
Alkene functional group gets priority over phenyl substituents and thus name is trans stilbene.
(h)
Interpretation:
The name of the given compound must be written.
Concept Introduction:
For benzene derivatives the major functional group gets priority and the other functional groups become substituents.
The name of the given compound is 2, 4-dichlorotoluene.
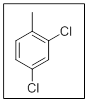
In the given structure there are two chloro substituents with respect to methyl group. Those are at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between chloro and methyl chloro gets priority over methyl due to alphabetical order. All the haloalkanes are named as substituted alkanes. Thus the name is2, 4-dichlorotoluene.
Explanation of Solution

In the given structure there are two chloro substituents with respect to methyl group. Those are at position
The priority order of different functional groups as per IUPAC nomenclature comes from the consideration of number of electronegative atoms in the functional group. More the number of electronegative atoms more are the priority of the functional group. Between chloro and methyl chloro gets priority over methyl due to alphabetical order. All the haloalkanes are named as substituted alkanes. Thus the name is2, 4-dichlorotoluene.
Want to see more full solutions like this?
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





