
Concept explainers
(a)
Interpretation: To identify the number of steps in glycolysis that produce ATP.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:
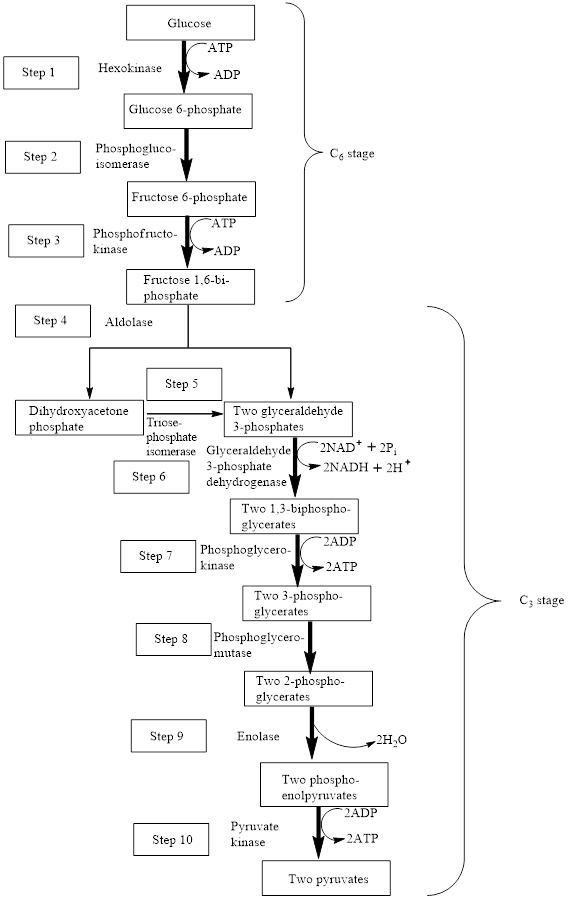
Adenosine triphosphate (ATP) is a molecule that is defined as the energy currency of life and provides energy to carry out the metabolic processes in the living cells. It is converted either to adenosine monophosphate (AMP) or to adenosine diphosphate (ADP) after the consumption in the metabolic processes.
(a)
Answer to Problem 13.17EP
In glycolysis, two steps produce ATP.
Explanation of Solution
In step 7 and step 10, two ATP molecules are produced in each step. Hence, in the glycolysis pathway, four ATP molecules are produced is two steps.
(b)
Interpretation: To identify the number of steps in glycolysis that involve phosphorylation.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
(b)
Answer to Problem 13.17EP
Phosphorylation is involved in five steps.
Explanation of Solution
The first step in the glycolysis process is the phosphorylation of glucose using ATP. Glucose is converted to
In the third step,
In step 6,
Therefore, step 6 proceeds through the oxidation and phosphorylation reaction using
The seventh step in the glycolysis process is the phosphorylation of
Step 10 is the final step of the glycolysis process. In step 10, phosphoenolpyruvate is converted to pyruvate using ADP, thus, ADP is converted to ATP. Pyruvate kinase enzymes are involved in this reaction. The type of reaction is phosphorylation. The word equation for the phosphorylation reaction is as follows:
Therefore, step 1, step 2, step 6, step 7 and step 10 involved the phosphorylation reaction.
(c)
Interpretation: To identify the number of steps in glycolysis that involve
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

A reactant is defined as the substance that is initially present in the
Nicotinamide adenine dinucleotide is associated with the
(c)
Answer to Problem 13.17EP
One step involves
Explanation of Solution
In step 6,
Therefore,
(d)
Interpretation: To identify the number of steps in glycolysis that involve a compound with a high-energy bond as a reactant.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
High energy compounds are those compounds that release a large amount of energy upon hydrolysis. These compounds consist of highly strained bonds that are responsible for the release of a high amount of energy. The compounds containing a phosphate group are examples of high energy compounds.
A high-energy phosphate group is formed when a phosphate group is attached to a carbon atom participating in carbon-oxygen or carbon-carbon double bond.
(d)
Answer to Problem 13.17EP
Two steps involve a compound with a high-energy bond as a reactant.
Explanation of Solution
The seventh step in the glycolysis process is the phosphorylation of
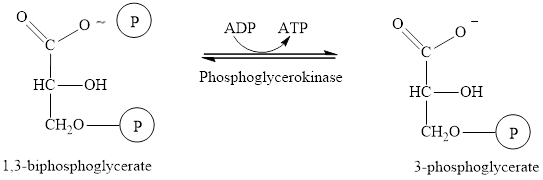
Here,  denotes the
denotes the
In step 10, phosphoenolpyruvate is converted to pyruvate using ADP, thus, ADP is converted to ATP. Pyruvate kinase enzymes are involved in this reaction. Phosphoenolpyruvate is a compound with a high-energy phosphate group because the phosphate group is attached to that carbon atom which is involved in carbon-carbon double bond. The chemical reaction is as follows:

Therefore, in step 10, a high-energy bond is encountered as a reactant.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic And Biological Chemistry
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



