
Concept explainers
Interpretation:
The missing reagents for each step in Your Turn 13.6 are to be supplied.
Concept introduction:
In order to identify the missing reagents in the given reaction sequence, it is important to identify if the reaction involves a functional group transformation, or it is a reaction that alters the carbon skeleton. In each given reaction, the bonds which are broken and formed are to be identified. The regioselectivity and stereochemistry play an important role in the outcome of the reaction when a specific reagent is used.
Under basic conditions, the nucleophile attacks the
Answer to Problem 13.1P
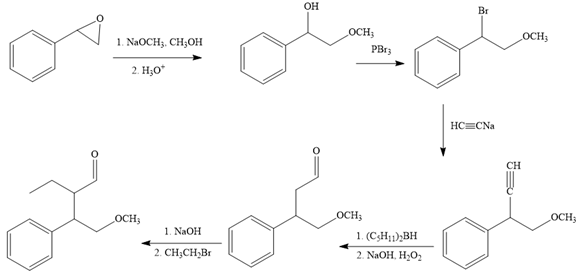
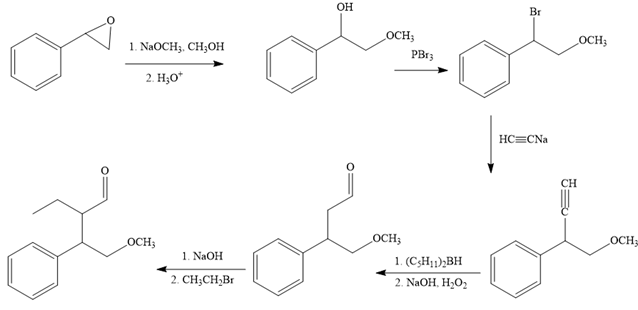
The missing reagents for each step in the given reaction sequence are given below:
Explanation of Solution
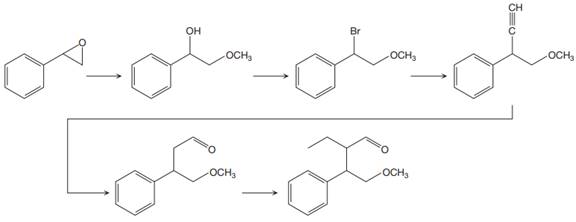
The reaction sequence given in Your Turn 13.6 is:
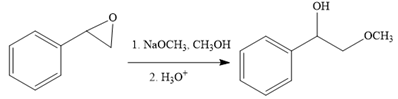
The first reaction is the conversion of an epoxide to alcohol. Thus, it is a functional group transformation reaction in which the nucleophile,
The first reaction and the missing reagents for it are shown below:
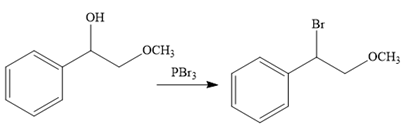
The second reaction also involves functional group transformation. The alcoholic (
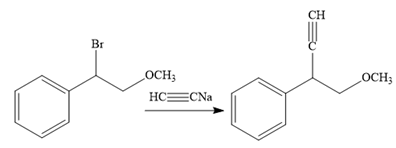
In the third reaction, the bromine atom is replaced by an acetylene group (
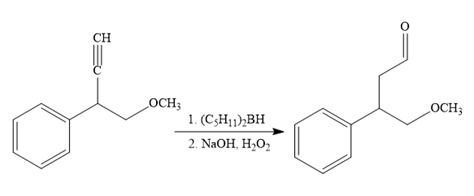
The fourth reaction in the given reaction sequence is a reaction involving a functional group transformation. Terminal alkynes undergo a hydroboration-oxidation reaction which leads to the formation of an aldehyde. The reagents used in the hydroboration-oxidation reaction are disiamylborane [
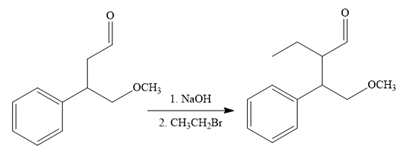
The fifth reaction involves the alteration of the carbon skeleton. The alpha hydrogen attached to an alpha carbon in aldehydes is weakly acidic. A strong base abstracts this alpha hydrogen to form an enolate ion. This enolate serves as a nucleophile and reacts with an alkyl halide via
The complete reaction sequence with appropriate reagents for each are given below:
In order to identify the missing reagents in the given reaction sequence, it is important to identify if the reaction involves a functional group transformation or it is a reaction that alters the carbon skeleton.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
