
Concept explainers
13-25 Answer true or false.
(a) Phenols and alcohols have in common the presence of an —OH group.
Phenols are weak acids and react with strong bases to give water-soluble salts.
- The pK„ of phenol is smaller than that of acetic acid.
(f, A characteristic of a chain initiation step is conversion of a nonradical to a radical.
(a)
Interpretation:
To analyse whether the given statement: Phenols and alcohols have in common the presence of an −OH group,is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
Phenols and alcohols have in common the presence of an −OH group. Thus, the statement is true.
Explanation of Solution
The alcohols are represented by a general formula: R-OH. In alcohols, the r is an alkyl group. The phenols are represented by a general formula: R-OH In phenol, the r is an aromatic benzene ring. An example of alcohol is methanol compared to phenol, as shown in the figure below:
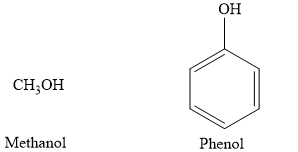
Therefore, the given statement is true.
(b)
Interpretation:
To analyse whether the given statement: Phenols are weak acids and react with strong bases to give water-soluble salts, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
Phenols are weak acids and react with strong bases to give water-soluble salts. Thus, the statement is true.
Explanation of Solution
Phenol is a weak acid. When it reacts with a strong base like sodium hydroxide NaOH, it forms water-soluble salt, or sodium phenoxide. Therefore, the given statement is true.
(c)
Interpretation:
To analyse whether the given statement: The
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
The
Explanation of Solution
The strength of an acid is measured by the acid dissociation constant Ka.
Consider this reaction.
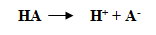
The acid dissociation constant is given by the formula.
The pKa is the logarithmic measurement of the acid dissociation constant. We know that the pKa value for phenol is 10 and for acetic acid is 4.7. Hence, the pKa value of phenol is much greater than the pKa value of acetic acid. Therefore, the given statement is false.
(d)
Interpretation:
To analyse whether the given statement: Autoxidation converts an R-H group to an R-OH group, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
Autoxidation converts an R-H group to an R-OOH group.Thus, the statement is false.
Explanation of Solution
Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. In auto-oxidation, the r — H group changes to an ROOH group and not to an ROH group. Therefore, the given statement is false.
(e)
Interpretation:
To analyze whether the given statement: A carbon radical has only seven electrons in the valence shell of one of its carbons, and this carbon bears a positive charge, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
A carbon radical has only seven electrons in the valence shell of one of its carbons, and this carbon may bear a positive charge or negative charge of no charge.Thus, the statement is false.
Explanation of Solution
Radicals are atoms, molecules, or ions with unpaired electrons or untilled electrons in their valence shells. A radical may have a positive charge, a negative charge, or no charge. A carbon radical does not carry any charge on it. Therefore, the given statement is false.
(f)
Interpretation:
To analyze whether the given statement -A characteristic of a chain initiation step is conversion of a nonradical to a radical, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
A characteristic of a chain initiation step is conversion of a nonradical to a radical. Thus, the statement is true.
Explanation of Solution
The characteristic of any chain initiation is the conversion of any non-radical to a radical. In a chain initiation process, hydrogen is converted to a hydrogen radical and carbon is converted to a carbon radical. For Example,
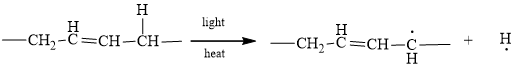
Therefore, the given statement is true.
(g)
Interpretation:
To analyze whether the given statement -Autoxidation is a radical-chain reaction, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
Autoxidation is a radical-chain reaction. Thus, the statement is true.
Explanation of Solution
Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a diradical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound. Therefore, the given statement is true.
(h)
Interpretation:
To analyze whether the given statement -A characteristic of the chain propagation step is reaction of a radical and a molecule to form a new radical and a new molecule, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Phenols act as antioxidants. Autoxidation is an oxidation reaction that requires only oxygen and no other reactants. It takes place in molecules containing carbon-carbon double bonds. Auto oxidation is a radical chain process. It takes place in different steps. In the first step, a non-radical compound changes to a radical form. For example, an oxygen atom is a di-radical. A radical reacts with oxygen to form a new radical. This new radical reacts with another molecule to form a new auto-oxidation product and a carbon radical compound.
Answer to Problem 13.25P
A characteristic of the chain propagation step is reaction of a radical and a molecule to form a new radical and a new molecule. Thus, the statement is true.
Explanation of Solution
A chain propagation step takes place in different steps. Autoxidation is an example of a chain propagation reaction. In the first step, a nonradical compound is changed to a radical form. Oxygen is a di-radical. A radical reacts with oxygen to form a new radical, which reacts with another radical and forms a carbon radical and a chain radical. Therefore, the given statement is true.
(i)
Interpretation:
To analyze whether the given statement -Vitamin E and other natural antioxidants function by interrupting the cycle of chain propagation steps that occurs in autoxidation, is true or not.
Concept Introduction:
Phenols are the compounds which have a hydroxyl group (—OH) bonded directly to a benzene ring. Phenol is a weak acid and pKa value for phenol is 10. Phenols are oxidized to quinone which is in turn easily reduced to hydroquinone.
Autoxidation is an oxidation reaction that requires only oxygen and no other reactants whereas the substance which inhibits oxidation is known as antioxidant.
Answer to Problem 13.25P
Vitamin E and other natural antioxidants function by interrupting the cycle of chain propagation steps that occurs in autoxidation. Thus the statement is true.
Explanation of Solution
Vitamin E is a stable radical. It breaks the chain propagation steps in auto-oxidation and prevents the formation of destructive hydro peroxides. Therefore, the given statement is true.
Want to see more full solutions like this?
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
- Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give each of the following alcohols as the major product. More than one alkene may give each compound as the major productarrow_forward13-57 Benzene, as we have seen in this chapter, is the simplest aromatic compound. Pyidine is an analog of benzene in which a CH group is replaced by a nitrogenarrow_forward12-50 Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give the indicated alcohol as the major product. More than one alkene may give each alcohol as the major product. 3-Hexanol 1-Methylcyclobutanol 2-Methyl-2-butanol 2-Propanolarrow_forward
- 14-78 Consider alkenes A, B, and C. each of which has the same molecular formula, C(.H12. Alkenes B and C can each be separated into cis and trans isomers. Upon catalytic reduction using H,, in the presence of a transition metal catalyst (Ni, Pd, or Pt>, alkenes A, B, and C all give hexane as the only product. Acid- catalyzed hydration of alkene C gives one alcohol with the molecular formula CeH14O. Acid catalyzed- hydration of alkene B gives an equal mixture of two alcohols, each with the molecular formula C6H14O. Acid-catalyzed hydration of alkene C gives only a single alcohol with the molecular formula C6H14O. Propose structural formulas for alkenes A, B, and C and the alcohols formed by acid-catalyzed hydration of each, consistent with these experimental results.arrow_forward17-27 Pentane, 1-butanol, and butanal all have approximately the same molecular weights but different boiling points. Arrange them in order of increasing boiling point. Explain the basis for your ranking.arrow_forward17-73 Alcohols can be prepared by the acid-catalyzed hydration of alkenes (Section 12-6B) and by the reduction of aldehydes and ketones (Section 17-4B). Show how you might prepare each of the following alcohols by (1) acid-catalyzed hydration of an alkene and (2) reduction of an aldehyde or a ketone. (a) Ethanol (b) Cyclohexanol (c) 2-Propanol (d) 1-Phenylethanolarrow_forward
- 17-26 Account for the fact that acetone has a higher boiling point (56°C) than ethyl methyl ether (11°C) even though their molecular weights are almost the same.arrow_forward13-20 Three products with the molecular formula CgH^BrCl form when bromobenzene is treated with chlorine, Cl2, in the presence of FeCl3 as a catalyst. Name and draw a structural formula for each product.arrow_forward14-49 Answer true or false. Today, the major carbon sources for the synthesis of methanol are coal and methane (natural gas), both nonrenewable resources. Today the major carbon sources for the synthesis of ethanol are petroleum and natural gas, both nonrenewable resources. Intermolecular acid-catalyzed dehydration of ethanol gives diethyl ether. Conversion of ethylene to ethylene glycol involves oxidation to ethylene oxide, followed by acid-catalyzed hydration (addition of water, to ethylene oxide. Ethylene glycol is soluble in water in all proportions. A major use of ethylene glycol is as automobile antifreeze.arrow_forward
- 13-19 Suppose you have unlabeled bottles of benzene and cyclohexene. What chemical reaction could you use to tell which bottle contains which chemical? Explain what you would do, what you would expect to see, and how you would explain your observations. Write an equation for a positive test.arrow_forward13-15 Draw structural formulas for these compounds l-Bromo-2-chloro-4-ethylbenzene 4-Bromo-l,2-dimethylbenzene 2,4,6-Trinitrotoluene 4-Phenyl-l-pentene p-Cresol (D 2,4-Dichlorophenolarrow_forward14-33 Write equations for the reaction of 1-butanol, a primary alcohol, with these reagents. H2SO4, heat K2Cr2O7, H2SO4arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

