
What products would result from the following processes?
Write an equation for each reaction.
a.
b.
c.
d.
e.
(a)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
No product is formed when
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The controlled oxidation of
No product is formed when
(b)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at temperature
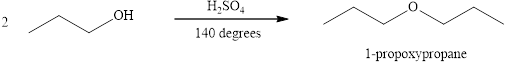
Figure 1
The product formed when
(c)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
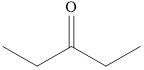
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
As
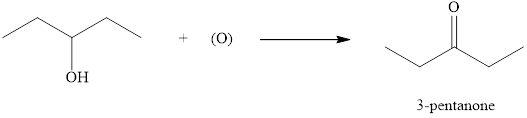
Figure 2
The product formed when
(d)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at temperature
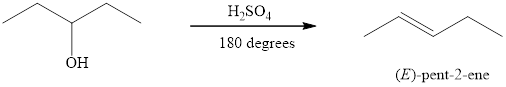
Figure 3
The product formed when
(e)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
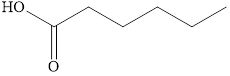
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Generally, primary alcohols on oxidation give aldehyde. However, in the presence of excess oxidizing agent, aldehyde further oxidizes to carboxylic acid. Therefore, the product formed when

Figure 4
The product formed when
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- What products would result from the following processes? Write an equation for each reaction. a. 1-Butanol is heated to 140C in the presence of sulfuric acid. b. 1-Butanol is subjected to an excess of oxidizing agent. c. 2-Pentanol is subjected to controlled oxidation. d. 2-Pentanol is heated to 180C in the presence of sulfuric acid. e. 2-Methyl-2-pentanol is subjected to controlled oxidation.arrow_forwardDraw the structures of the ethers that can be produced from the following alcohols: a. CH3CH2CH2OH b. c.arrow_forwardWHich of the following uses of ethers is not correct a. diethyl ether is used as a propellant for aerosol sprays b. ethers are used as an inert solvent c. solutes for varnishes and lacquers d. ethers used as cooling agentarrow_forward
- Kinetics and thermodynamics H+ catatylzes the esterification reaction of alcohols with carboxylic acids. Describe at least two ways the addition of H+ can increase the rate of the reaction. based on the imagearrow_forwardDESCRIPTION Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, ??=??. Alcohol can be synthesized by the reduction of aldehydes and ketones. The reduction of formaldehyde forms methanol while the other aldehydes produce primary alcohols. Moreover, secondary alcohols can be produced from the reduction of ketones. Aldehydes and ketones can be reduced by hydride agents’ reaction, catalytic hydrogenation and Grignard reagents reduction. Heterogeneous catalytic hydrogenation is the simplest method for the reduction of aldehydes and ketones into the corresponding alcohol, which is in huge demand. At present, metal catalysts are widely used in industry due to their high activity and low cost. TASK As a process engineer in a chemical manufacturing company, your team has been assigned to prepare the proposal on the process and suitable design of a reactor for catalytic hydrogenation of liquid aldehyde OR ketone into the desired alcohol based on the current…arrow_forwardWhat reagents are used in the esterification of Alcohols and Phenols? a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloridearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





