
Concept explainers
How many hydrogen atoms are present in a molecule of each of the compounds in Problem 13-26?
- a. 2-methylcyclopentene
- b. 1,3-cyclopentadiene
- c. 2,3-dimethylpentane
- d. 1-ethyl-2-methylcyclohexene
(a)
Interpretation:
The total number of hydrogen atoms present in the given molecule has to be identified.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Alkanes are a class of saturated hydrocarbons that do not contain a ring of carbon atoms but a chain of carbon atoms with carbon‑carbon single bonds. The general molecular formula for alkanes is
Alkenes and cycloalkenes are a class of unsaturated hydrocarbons that contain at least one double bond in its structure. The general molecular formula for alkene with one double bond is
Answer to Problem 13.28EP
The total number of hydrogen atoms present is 10.
Explanation of Solution
Cycloalkenes are unsaturated hydrocarbons that contain at least one double bond between carbon atoms with ring structure. The general molecular formula for cycloalkene with one double bond is

Carbon atoms are present at the intersection and at the end points. The above structure has five intersections and one end point. Therefore, there is a total of six carbon atoms. The total number of hydrogen atoms can be found by substituting in the general molecular formula as shown below,
The total number of hydrogen atoms that will be present in the given cycloalkene is found to be ten.
The total number of hydrogen atoms present in the molecule is ten.
(b)
Interpretation:
The total number of hydrogen atoms present in the given molecule has to be identified.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Alkanes are a class of saturated hydrocarbons that do not contain a ring of carbon atoms but a chain of carbon atoms with carbon‑carbon single bonds. The general molecular formula for alkanes is
Alkenes and cycloalkenes are a class of unsaturated hydrocarbons that contain at least one double bond in its structure. The general molecular formula for alkene with one double bond is
Answer to Problem 13.28EP
The total number of hydrogen atoms present is 6.
Explanation of Solution
Cycloalkenes are unsaturated hydrocarbons that contain at least one double bond between carbon atoms with a ring structure. The general molecular formula for cycloalkene with two double bond is
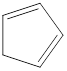
Carbon atoms are present at the intersection and at the end points. The above structure has five intersections and no end points. Therefore, there is a total of five carbon atoms. The total number of hydrogen atoms can be found by substituting in the general molecular formula as shown below,
The total number of hydrogen atoms that will be present in the given cycloalkene is found to be six.
The total number of hydrogen atoms present in the molecule is six.
(c)
Interpretation:
The total number of hydrogen atoms present in the given molecule has to be identified.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Alkanes are a class of saturated hydrocarbons that do not contain a ring of carbon atoms but a chain of carbon atoms with carbon‑carbon single bonds. The general molecular formula for alkanes is
Alkenes and cycloalkenes are a class of unsaturated hydrocarbons that contain at least one double bond in its structure. The general molecular formula for alkene with one double bond is
Answer to Problem 13.28EP
The total number of hydrogen atoms present is 16.
Explanation of Solution
Alkanes are saturated hydrocarbons that contain only single bonds between carbon atoms with no ring structure. The general molecular formula for alkane is
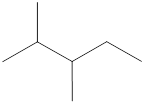
Carbon atoms are present at the intersection and at the end points. The above structure has three intersections and four end points. Therefore, there is a total of seven carbon atoms. The total number of hydrogen atoms can be found by substituting in the general molecular formula as shown below,
The total number of hydrogen atoms that will be present in the given alkane is found to be sixteen.
The total number of hydrogen atoms present in the molecule is sixteen.
(d)
Interpretation:
The total number of hydrogen atoms present in the given molecule has to be identified.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Alkanes are a class of saturated hydrocarbons that do not contain a ring of carbon atoms but a chain of carbon atoms with carbon‑carbon single bonds. The general molecular formula for alkanes is
Alkenes and cycloalkenes are a class of unsaturated hydrocarbons that contain at least one double bond in its structure. The general molecular formula for alkene with one double bond is
Answer to Problem 13.28EP
The total number of hydrogen atoms present is 16.
Explanation of Solution
Cycloalkenes are unsaturated hydrocarbons that contain at least one double bond between carbon atoms with ring structure. The general molecular formula for cycloalkene with one double bond is
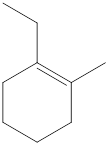
Carbon atoms are present at the intersection and at the end points. The above structure has seven intersections and two end points. Therefore, there is a total of nine carbon atoms. The total number of hydrogen atoms can be found by substituting in the general molecular formula as shown below,
The total number of hydrogen atoms that will be present in the given cycloalkene is found to be sixteen.
The total number of hydrogen atoms present in the molecule is sixteen.
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry
- How many hydrogen atoms are present in a molecule of each of the compounds in Problem 13-26? a. 2-methylcyclopentene b. 1,3-cyclopentadiene c. 2,3-dimethylpentane d. 1-ethyl-2-methylcyclohexenearrow_forward12-59 Show how to convert 1-butene to these compounds, (a) Butane(b) 2-Butanol (c) 2-Bromobutane(d) 1,2-Dibromobutanearrow_forwardIndicate whether each statement is true or false. Two generic isomers of pentane are n-pentane and neo-pentane. Alkenes can have cis and trans isomers around the CC double bond. Alkynes can have cis and trans isomers around CC triple bond.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





