
Concept explainers
An unknown compound has the molecular formula C 9 H 11 B r. Its proton NMR spectrum shows the following absorptions:
singlet, δ 7.1, integral 44 mm
singlet, δ 2.3, integral 130 mm
singlet, δ 2.2, integral 67 mm
Propose a structure for this compound.
Interpretation:
The structure of the given unknown compound showing the given absorptions in the NMR spectrum is to be proposed.
Concept introduction:
NMR spectroscopy is a technique used to determine the unique structure of the compounds. It identifies the carbon-hydrogen bonding of an organic compound. A hydrogen atom is called as a proton in the NMR spectroscopy.
Answer to Problem 13.33SP
The structure of the given unknown compound showing the given absorptions in the NMR spectrum is given below.
Explanation of Solution
The molecular formula of the compound (
The double bond equivalent is calculated by the formula,
Substitute the given values in the above expression.
The DBE value is more than three and one signal present at
The other signals present in the NMR spectrum shows that,
From the above data analysis of
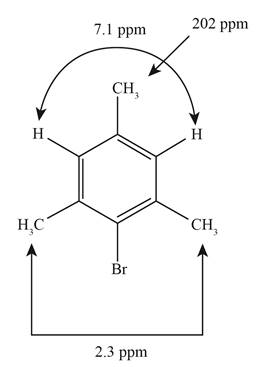
Figure 1
The structure of the given unknown compound showing the given absorptions in the NMR spectrum has been rightfully stated.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
- Propose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forwardPropose a structure consistent with the following spectral data for a compound C8H18O2: IR: 3350 cm-1 1H NMR: 1.24 δ (12 H, singlet); 1.56 δ (4 H, singlet); 1.95 δ (2 H, singlet)arrow_forwardCompound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forward
- A 13C NMR spectrum of commercially available 2,4-pentanediol, shows five peaks at 23.3, 23.9, 46.5, 64.8, and 68.1 . Explain.arrow_forwardPropose a structural formula for each compound consistent with its 1H-NMR and 13C-NMR spectra. (a) C5H10O2 (b) C7H14O2 (c) C6 H12O2 (d) C7H12O4 (e) C4H7ClO2 (f) C4H6O2arrow_forwardThe compound has a molecular formula of C5H10O and the following 13C spectrum. A proton NMR was measured, but the data was lost except for the fact that it showed three distinct signals: a singlet, a doublet, and a septet, all of which appear between 0 and 3 ppm. Identify the compound, and draw its 1H NMR.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

