
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
14th Edition
ISBN: 9781259327933
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.37QP
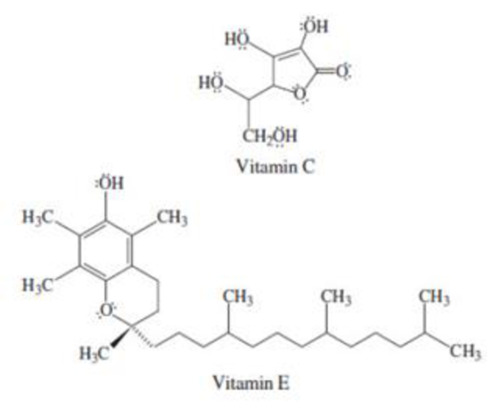
The difference between water-soluble and fat-soluble vitamins is their molecular structures. Water-soluble vitamins tend to have multiple polar groups that can interact with water to form hydrogen bonds, whereas frit-soluble vitamins tend to be nonpolar molecules consisting predominately of hydrocarbon chains. This is an example of what is meant by the expression “like dissolves like.” Predict whether vitamin C and vitamin E will be water soluble or fat soluble.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
Ch. 13.2 - Determine for each solute whether the solubility...Ch. 13.2 - Predict whether iodine (I2) is more soluble in...Ch. 13.2 - Prob. 1PPBCh. 13.2 - Prob. 1PPCCh. 13.2 - Prob. 13.2.1SRCh. 13.2 - Prob. 13.2.2SRCh. 13.3 - Prob. 13.2WECh. 13.3 - Determine (a) the molality and (b) the percent by...Ch. 13.3 - Prob. 2PPBCh. 13.3 - Prob. 2PPC
Ch. 13.3 - Rubbing alcohol is a mixture of isopropyl alcohol...Ch. 13.3 - Prob. 3PPACh. 13.3 - Prob. 3PPBCh. 13.3 - Prob. 3PPCCh. 13.3 - Prob. 13.3.1SRCh. 13.3 - Prob. 13.3.2SRCh. 13.3 - Prob. 13.3.3SRCh. 13.3 - Prob. 13.3.4SRCh. 13.4 - Calculate the concentration of carbon dioxide in a...Ch. 13.4 - Calculate the concentration of CO2 in water at 25C...Ch. 13.4 - Prob. 4PPBCh. 13.4 - Prob. 4PPCCh. 13.4 - Prob. 13.4.1SRCh. 13.4 - Prob. 13.4.2SRCh. 13.5 - Prob. 13.5WECh. 13.5 - Calculate the vapor pressure of a solution made by...Ch. 13.5 - Calculate the mass of urea that should be...Ch. 13.5 - The diagrams [(i)(iv)] represent four closed...Ch. 13.5 - Ethylene glycol [CH2(OH)CH2(OH)] is a common...Ch. 13.5 - Prob. 6PPACh. 13.5 - What mass of ethylene glycol must be added to 1525...Ch. 13.5 - Prob. 6PPCCh. 13.5 - Prob. 13.7WECh. 13.5 - Prob. 7PPACh. 13.5 - Prob. 7PPBCh. 13.5 - Prob. 7PPCCh. 13.5 - Prob. 13.5.1SRCh. 13.5 - Prob. 13.5.2SRCh. 13.5 - Prob. 13.5.3SRCh. 13.5 - Prob. 13.5.4SRCh. 13.6 - Quinine was the first drug widely used to treat...Ch. 13.6 - Prob. 8PPACh. 13.6 - Prob. 8PPBCh. 13.6 - Prob. 8PPCCh. 13.6 - Prob. 13.9WECh. 13.6 - A solution made by dissolving 25 mg of insulin in...Ch. 13.6 - What mass of insulin must be dissolved in 50.0 mL...Ch. 13.6 - The first diagram represents one aqueous solution...Ch. 13.6 - Prob. 13.10WECh. 13.6 - An aqueous solution that is 0.0100 M in acetic...Ch. 13.6 - Prob. 10PPBCh. 13.6 - Prob. 10PPCCh. 13.6 - Prob. 13.6.1SRCh. 13.6 - Prob. 13.6.2SRCh. 13 - Prob. 13.1QPCh. 13 - Prob. 13.2QPCh. 13 - Prob. 13.3QPCh. 13 - Prob. 13.4QPCh. 13 - Prob. 13.5QPCh. 13 - Prob. 13.6QPCh. 13 - Explain why dissolving a solid almost always leads...Ch. 13 - Describe the factors that affect the solubility of...Ch. 13 - Prob. 13.9QPCh. 13 - Prob. 13.10QPCh. 13 - Arrange the following compounds in order of...Ch. 13 - Prob. 13.12QPCh. 13 - Prob. 13.13QPCh. 13 - Prob. 13.14QPCh. 13 - Prob. 13.15QPCh. 13 - Prob. 13.16QPCh. 13 - Prob. 13.17QPCh. 13 - Prob. 13.18QPCh. 13 - Prob. 13.19QPCh. 13 - Prob. 13.20QPCh. 13 - Prob. 13.21QPCh. 13 - Prob. 13.22QPCh. 13 - Prob. 13.23QPCh. 13 - Prob. 13.24QPCh. 13 - Fish breathe the dissolved air in water through...Ch. 13 - Prob. 13.26QPCh. 13 - Discuss the factors that influence the solubility...Ch. 13 - What is thermal pollution? Why is it harmful to...Ch. 13 - Prob. 13.29QPCh. 13 - A student is observing two beakers of water. One...Ch. 13 - A man bought a goldfish in a pet shop. Upon...Ch. 13 - A 3.20-g sample of a salt dissolves in 9.10 g of...Ch. 13 - The solubility of KNO3 is 155 g per 100 g of water...Ch. 13 - Prob. 13.34QPCh. 13 - The solubility of CO2 in water at 25C and 1 atm is...Ch. 13 - The solubility of N2 in blood at 37C and at a...Ch. 13 - The difference between water-soluble and...Ch. 13 - Predict whether each vitamin will be water soluble...Ch. 13 - Prob. 13.39QPCh. 13 - The first diagram represents an open system with...Ch. 13 - The diagrams represent an aqueous solution at two...Ch. 13 - Prob. 13.42QPCh. 13 - Prob. 13.43QPCh. 13 - Write the equation representing Raoults law, and...Ch. 13 - Prob. 13.45QPCh. 13 - Write the equations relating boiling-point...Ch. 13 - Prob. 13.47QPCh. 13 - Prob. 13.48QPCh. 13 - What is osmosis? What is a semipermeable membrane?Ch. 13 - Prob. 13.50QPCh. 13 - Prob. 13.51QPCh. 13 - Prob. 13.52QPCh. 13 - What are ion pairs? What effect does ion-pair...Ch. 13 - Prob. 13.54QPCh. 13 - Prob. 13.55QPCh. 13 - Prob. 13.56QPCh. 13 - Prob. 13.57QPCh. 13 - The vapor pressure of benzene is 100.0 mmHg at...Ch. 13 - The vapor pressures of ethanol (C2H5OH) and...Ch. 13 - The vapor pressure of ethanol (C2H5OH) at 20C is...Ch. 13 - Prob. 13.61QPCh. 13 - What arc the boiling point and freezing point of a...Ch. 13 - Prob. 13.63QPCh. 13 - How many liters of the antifreeze ethylene glycol...Ch. 13 - Prob. 13.65QPCh. 13 - Prob. 13.66QPCh. 13 - What are the normal freezing points and boiling...Ch. 13 - At 25C, the vapor pressure of pure water is 23.76...Ch. 13 - Both NaCl and CaCl2 are used to melt ice on roads...Ch. 13 - A 0.86 percent by mass solution of NaCl is called...Ch. 13 - Prob. 13.71QPCh. 13 - Calculate the osmotic pressure of a 0.0500 M MgSO4...Ch. 13 - The tallest trees known are the redwoods in...Ch. 13 - Calculate the difference in osmotic pressure (in...Ch. 13 - Prob. 13.75QPCh. 13 - Consider two aqueous solutions, one of sucrose...Ch. 13 - Arrange the following solutions in order of...Ch. 13 - Prob. 13.78QPCh. 13 - Indicate which compound in each of the following...Ch. 13 - Describe how you would use freezing-point...Ch. 13 - Prob. 13.81QPCh. 13 - The elemental analysis of an organic solid...Ch. 13 - A solution of 2.50 g of a compound having the...Ch. 13 - The molar mass of benzoic acid (C6H5COOH)...Ch. 13 - A solution containing 0.8330 g of a polymer of...Ch. 13 - Prob. 13.86QPCh. 13 - A solution of 6.S5 g of a carbohydrate m 100.0 g...Ch. 13 - A 0.036-M aqueous nitrous acid (HNO2) solution has...Ch. 13 - Prob. 13.89QPCh. 13 - Prob. 13.90QPCh. 13 - Prob. 13.91QPCh. 13 - Prob. 13.92QPCh. 13 - Prob. 13.93QPCh. 13 - Lysozyme is an enzyme that cleaves bacterial cell...Ch. 13 - The blood sugar (glucose) level of a diabetic...Ch. 13 - Trees in cold climates may be subjected to...Ch. 13 - Prob. 13.97QPCh. 13 - Two liquids A and B have vapor pressures of 76 and...Ch. 13 - Determine the van't Hoff factor of Na3PO4 in a...Ch. 13 - Prob. 13.100QPCh. 13 - Consider the three mercury manometers shown in the...Ch. 13 - A forensic chemist is given a white powder for...Ch. 13 - Prob. 13.103QPCh. 13 - A solution of 1.00 g of anhydrous aluminum...Ch. 13 - Explain why reverse osmosis is (theoretically)...Ch. 13 - What masses of sodium chloride, magnesium...Ch. 13 - The osmotic pressure of blood plasma is...Ch. 13 - Prob. 13.108QPCh. 13 - A protein has been isolated as a salt with the...Ch. 13 - A nonvolatile organic compound Z was used to make...Ch. 13 - Prob. 13.111QPCh. 13 - State which of the alcohols listed in Problem...Ch. 13 - Before a carbonated beverage bottle is sealed, it...Ch. 13 - Iodine (I2) is only sparingly soluble in water...Ch. 13 - (a) The root cells of plants contain a solution...Ch. 13 - Hemoglobin, the oxygen-transport protein, binds...Ch. 13 - Prob. 13.117QPCh. 13 - In the apparatus shown, what will happen if the...Ch. 13 - Concentrated hydrochloric acid is usually...Ch. 13 - Explain each of the following statements: (a) The...Ch. 13 - Prob. 13.121QPCh. 13 - A 1.32-g sample of a mixture of cyclohexane...Ch. 13 - How does each of the following affect the...Ch. 13 - A solution contains two volatile liquids A and B....Ch. 13 - Prob. 13.125QPCh. 13 - A mixture of ethanol and 1-propanol behaves...Ch. 13 - Ammonia (NH3) is very soluble in water, but...Ch. 13 - For ideal solutions, the volumes are additive....Ch. 13 - Acetic acid is a weak acid that ionizes in...Ch. 13 - Which vitamins (sec the given structures) do you...Ch. 13 - Calculate the percent by mass of the solute in...Ch. 13 - Acetic acid is a polar molecule and can form...Ch. 13 - Prob. 13.133QPCh. 13 - Fish in the Antarctic Ocean swim in water at about...Ch. 13 - Why are ice cubes (e.g., those you see in the...Ch. 13 - Prob. 13.136QPCh. 13 - Two beakers are placed in a closed container...Ch. 13 - (a) Derive the equation relating the molality (m)...Ch. 13 - At 27C, the vapor pressure of pure water is 23.76...Ch. 13 - A very long pipe is capped at one end with a...Ch. 13 - A mixture of liquids A and B exhibits ideal...Ch. 13 - Use Henrys law and the ideal gas equation to prove...Ch. 13 - Prob. 13.143QPCh. 13 - Prob. 13.144QPCh. 13 - The diagram here shows vapor pressure curves for...Ch. 13 - Valinomycin is an antibiotic. It functions by...Ch. 13 - Prob. 13.147QPCh. 13 - Prob. 13.148QPCh. 13 - Prob. 13.149QPCh. 13 - Explain why we cannot use osmotic pressure to...Ch. 13 - Which of the following processes is accompanied by...Ch. 13 - For each of the processes depicted here, determine...Ch. 13 - For each of the processes depicted here, determine...Ch. 13 - Prob. 13.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rationalize the temperature dependence of the solubility of a gas in water in terms of the kinetic molecular theory.arrow_forwardWhat would be the freezing point of a solution formed by adding 1.0 mole of glucose (a molecular compound) to the following amounts of water? a. 250 g (0.25 kg) b. 500 g (0.500 kg) c. 1000 g (1.000 kg) d. 2000 g (2.000 kg)arrow_forward
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY