
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
14th Edition
ISBN: 9781259327933
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.41QP
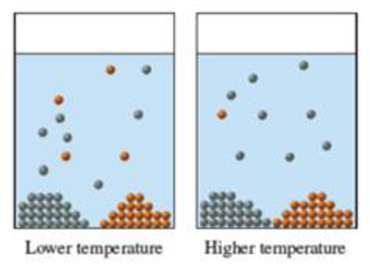
The diagrams represent an aqueous solution at two different temperatures. In both diagrams, the solution is saturated in two different solutes, each of which is represented by a different color. Using the diagrams, determine for each solute whether the dissolution process is endothermic or exothermic. For which of the solutes do you think the numerical value of ΔHsoln is greater? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
Ch. 13.2 - Determine for each solute whether the solubility...Ch. 13.2 - Predict whether iodine (I2) is more soluble in...Ch. 13.2 - Prob. 1PPBCh. 13.2 - Prob. 1PPCCh. 13.2 - Prob. 13.2.1SRCh. 13.2 - Prob. 13.2.2SRCh. 13.3 - Prob. 13.2WECh. 13.3 - Determine (a) the molality and (b) the percent by...Ch. 13.3 - Prob. 2PPBCh. 13.3 - Prob. 2PPC
Ch. 13.3 - Rubbing alcohol is a mixture of isopropyl alcohol...Ch. 13.3 - Prob. 3PPACh. 13.3 - Prob. 3PPBCh. 13.3 - Prob. 3PPCCh. 13.3 - Prob. 13.3.1SRCh. 13.3 - Prob. 13.3.2SRCh. 13.3 - Prob. 13.3.3SRCh. 13.3 - Prob. 13.3.4SRCh. 13.4 - Calculate the concentration of carbon dioxide in a...Ch. 13.4 - Calculate the concentration of CO2 in water at 25C...Ch. 13.4 - Prob. 4PPBCh. 13.4 - Prob. 4PPCCh. 13.4 - Prob. 13.4.1SRCh. 13.4 - Prob. 13.4.2SRCh. 13.5 - Prob. 13.5WECh. 13.5 - Calculate the vapor pressure of a solution made by...Ch. 13.5 - Calculate the mass of urea that should be...Ch. 13.5 - The diagrams [(i)(iv)] represent four closed...Ch. 13.5 - Ethylene glycol [CH2(OH)CH2(OH)] is a common...Ch. 13.5 - Prob. 6PPACh. 13.5 - What mass of ethylene glycol must be added to 1525...Ch. 13.5 - Prob. 6PPCCh. 13.5 - Prob. 13.7WECh. 13.5 - Prob. 7PPACh. 13.5 - Prob. 7PPBCh. 13.5 - Prob. 7PPCCh. 13.5 - Prob. 13.5.1SRCh. 13.5 - Prob. 13.5.2SRCh. 13.5 - Prob. 13.5.3SRCh. 13.5 - Prob. 13.5.4SRCh. 13.6 - Quinine was the first drug widely used to treat...Ch. 13.6 - Prob. 8PPACh. 13.6 - Prob. 8PPBCh. 13.6 - Prob. 8PPCCh. 13.6 - Prob. 13.9WECh. 13.6 - A solution made by dissolving 25 mg of insulin in...Ch. 13.6 - What mass of insulin must be dissolved in 50.0 mL...Ch. 13.6 - The first diagram represents one aqueous solution...Ch. 13.6 - Prob. 13.10WECh. 13.6 - An aqueous solution that is 0.0100 M in acetic...Ch. 13.6 - Prob. 10PPBCh. 13.6 - Prob. 10PPCCh. 13.6 - Prob. 13.6.1SRCh. 13.6 - Prob. 13.6.2SRCh. 13 - Prob. 13.1QPCh. 13 - Prob. 13.2QPCh. 13 - Prob. 13.3QPCh. 13 - Prob. 13.4QPCh. 13 - Prob. 13.5QPCh. 13 - Prob. 13.6QPCh. 13 - Explain why dissolving a solid almost always leads...Ch. 13 - Describe the factors that affect the solubility of...Ch. 13 - Prob. 13.9QPCh. 13 - Prob. 13.10QPCh. 13 - Arrange the following compounds in order of...Ch. 13 - Prob. 13.12QPCh. 13 - Prob. 13.13QPCh. 13 - Prob. 13.14QPCh. 13 - Prob. 13.15QPCh. 13 - Prob. 13.16QPCh. 13 - Prob. 13.17QPCh. 13 - Prob. 13.18QPCh. 13 - Prob. 13.19QPCh. 13 - Prob. 13.20QPCh. 13 - Prob. 13.21QPCh. 13 - Prob. 13.22QPCh. 13 - Prob. 13.23QPCh. 13 - Prob. 13.24QPCh. 13 - Fish breathe the dissolved air in water through...Ch. 13 - Prob. 13.26QPCh. 13 - Discuss the factors that influence the solubility...Ch. 13 - What is thermal pollution? Why is it harmful to...Ch. 13 - Prob. 13.29QPCh. 13 - A student is observing two beakers of water. One...Ch. 13 - A man bought a goldfish in a pet shop. Upon...Ch. 13 - A 3.20-g sample of a salt dissolves in 9.10 g of...Ch. 13 - The solubility of KNO3 is 155 g per 100 g of water...Ch. 13 - Prob. 13.34QPCh. 13 - The solubility of CO2 in water at 25C and 1 atm is...Ch. 13 - The solubility of N2 in blood at 37C and at a...Ch. 13 - The difference between water-soluble and...Ch. 13 - Predict whether each vitamin will be water soluble...Ch. 13 - Prob. 13.39QPCh. 13 - The first diagram represents an open system with...Ch. 13 - The diagrams represent an aqueous solution at two...Ch. 13 - Prob. 13.42QPCh. 13 - Prob. 13.43QPCh. 13 - Write the equation representing Raoults law, and...Ch. 13 - Prob. 13.45QPCh. 13 - Write the equations relating boiling-point...Ch. 13 - Prob. 13.47QPCh. 13 - Prob. 13.48QPCh. 13 - What is osmosis? What is a semipermeable membrane?Ch. 13 - Prob. 13.50QPCh. 13 - Prob. 13.51QPCh. 13 - Prob. 13.52QPCh. 13 - What are ion pairs? What effect does ion-pair...Ch. 13 - Prob. 13.54QPCh. 13 - Prob. 13.55QPCh. 13 - Prob. 13.56QPCh. 13 - Prob. 13.57QPCh. 13 - The vapor pressure of benzene is 100.0 mmHg at...Ch. 13 - The vapor pressures of ethanol (C2H5OH) and...Ch. 13 - The vapor pressure of ethanol (C2H5OH) at 20C is...Ch. 13 - Prob. 13.61QPCh. 13 - What arc the boiling point and freezing point of a...Ch. 13 - Prob. 13.63QPCh. 13 - How many liters of the antifreeze ethylene glycol...Ch. 13 - Prob. 13.65QPCh. 13 - Prob. 13.66QPCh. 13 - What are the normal freezing points and boiling...Ch. 13 - At 25C, the vapor pressure of pure water is 23.76...Ch. 13 - Both NaCl and CaCl2 are used to melt ice on roads...Ch. 13 - A 0.86 percent by mass solution of NaCl is called...Ch. 13 - Prob. 13.71QPCh. 13 - Calculate the osmotic pressure of a 0.0500 M MgSO4...Ch. 13 - The tallest trees known are the redwoods in...Ch. 13 - Calculate the difference in osmotic pressure (in...Ch. 13 - Prob. 13.75QPCh. 13 - Consider two aqueous solutions, one of sucrose...Ch. 13 - Arrange the following solutions in order of...Ch. 13 - Prob. 13.78QPCh. 13 - Indicate which compound in each of the following...Ch. 13 - Describe how you would use freezing-point...Ch. 13 - Prob. 13.81QPCh. 13 - The elemental analysis of an organic solid...Ch. 13 - A solution of 2.50 g of a compound having the...Ch. 13 - The molar mass of benzoic acid (C6H5COOH)...Ch. 13 - A solution containing 0.8330 g of a polymer of...Ch. 13 - Prob. 13.86QPCh. 13 - A solution of 6.S5 g of a carbohydrate m 100.0 g...Ch. 13 - A 0.036-M aqueous nitrous acid (HNO2) solution has...Ch. 13 - Prob. 13.89QPCh. 13 - Prob. 13.90QPCh. 13 - Prob. 13.91QPCh. 13 - Prob. 13.92QPCh. 13 - Prob. 13.93QPCh. 13 - Lysozyme is an enzyme that cleaves bacterial cell...Ch. 13 - The blood sugar (glucose) level of a diabetic...Ch. 13 - Trees in cold climates may be subjected to...Ch. 13 - Prob. 13.97QPCh. 13 - Two liquids A and B have vapor pressures of 76 and...Ch. 13 - Determine the van't Hoff factor of Na3PO4 in a...Ch. 13 - Prob. 13.100QPCh. 13 - Consider the three mercury manometers shown in the...Ch. 13 - A forensic chemist is given a white powder for...Ch. 13 - Prob. 13.103QPCh. 13 - A solution of 1.00 g of anhydrous aluminum...Ch. 13 - Explain why reverse osmosis is (theoretically)...Ch. 13 - What masses of sodium chloride, magnesium...Ch. 13 - The osmotic pressure of blood plasma is...Ch. 13 - Prob. 13.108QPCh. 13 - A protein has been isolated as a salt with the...Ch. 13 - A nonvolatile organic compound Z was used to make...Ch. 13 - Prob. 13.111QPCh. 13 - State which of the alcohols listed in Problem...Ch. 13 - Before a carbonated beverage bottle is sealed, it...Ch. 13 - Iodine (I2) is only sparingly soluble in water...Ch. 13 - (a) The root cells of plants contain a solution...Ch. 13 - Hemoglobin, the oxygen-transport protein, binds...Ch. 13 - Prob. 13.117QPCh. 13 - In the apparatus shown, what will happen if the...Ch. 13 - Concentrated hydrochloric acid is usually...Ch. 13 - Explain each of the following statements: (a) The...Ch. 13 - Prob. 13.121QPCh. 13 - A 1.32-g sample of a mixture of cyclohexane...Ch. 13 - How does each of the following affect the...Ch. 13 - A solution contains two volatile liquids A and B....Ch. 13 - Prob. 13.125QPCh. 13 - A mixture of ethanol and 1-propanol behaves...Ch. 13 - Ammonia (NH3) is very soluble in water, but...Ch. 13 - For ideal solutions, the volumes are additive....Ch. 13 - Acetic acid is a weak acid that ionizes in...Ch. 13 - Which vitamins (sec the given structures) do you...Ch. 13 - Calculate the percent by mass of the solute in...Ch. 13 - Acetic acid is a polar molecule and can form...Ch. 13 - Prob. 13.133QPCh. 13 - Fish in the Antarctic Ocean swim in water at about...Ch. 13 - Why are ice cubes (e.g., those you see in the...Ch. 13 - Prob. 13.136QPCh. 13 - Two beakers are placed in a closed container...Ch. 13 - (a) Derive the equation relating the molality (m)...Ch. 13 - At 27C, the vapor pressure of pure water is 23.76...Ch. 13 - A very long pipe is capped at one end with a...Ch. 13 - A mixture of liquids A and B exhibits ideal...Ch. 13 - Use Henrys law and the ideal gas equation to prove...Ch. 13 - Prob. 13.143QPCh. 13 - Prob. 13.144QPCh. 13 - The diagram here shows vapor pressure curves for...Ch. 13 - Valinomycin is an antibiotic. It functions by...Ch. 13 - Prob. 13.147QPCh. 13 - Prob. 13.148QPCh. 13 - Prob. 13.149QPCh. 13 - Explain why we cannot use osmotic pressure to...Ch. 13 - Which of the following processes is accompanied by...Ch. 13 - For each of the processes depicted here, determine...Ch. 13 - For each of the processes depicted here, determine...Ch. 13 - Prob. 13.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which solute has the greatest effect on the boiling pointof 1.00 kg of water: 50.0 g of strontium chloride (SrCl2) or 150.0 g of carbon tetrachloride (CCl4) ? Justify youranswer.arrow_forwardIn a mountainous location, the boiling point of pure water is found to be 95C. How many grams of sodium chloride must be added to 1 kg of water to bring the boiling point back to 100C? Assume that i = 2.arrow_forwardTwo samples of sodium chloride solutions are brought to a boil on a stove. One of the solutions boils at 100.10C and the other at 100.15C. a Which of the solutions is more concentrated? b Which of the solutions would have a lower freezing point? c If you split the solution that boils at 100.1C into two portions, how would the boiling points of the samples compare? Which of the following statements do you agree with regarding the determination of your answer for part c? I. The question cannot be answered with certainty without knowing the volumes of each portion. II. Making the necessary assumption that the two samples have equal volumes, I was able to correctly answer the question. III. The volumes that the sample was split into are irrelevant when determining the correct answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY