
Concept explainers
What major IR absorptions are present above
a. 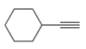 b.
b. 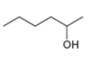 c.
c. 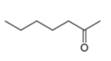 d.
d. 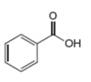
(a)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorption peaks are observed for
Explanation of Solution
The given compound is ethynylcyclohexane as shown below.
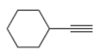
Figure 1
It contains
The major IR absorption peaks are observed for
(b)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hexan-2-ol as shown below.
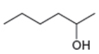
Figure 2
It contains
The major IR absorptions are observed for
(c)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hept-2-one as shown below.
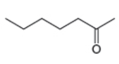
Figure 3
It contains
The major IR absorptions are observed for
(d)
Interpretation: The major IR absorptions are present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is benzoic acid as shown below.
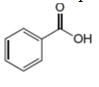
Figure 4
It contains,
The major IR absorptions are observed for
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry: Atoms First
Organic Chemistry (8th Edition)
Organic Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- Give a detailed explanation on how an NMR spectra is interpreted.arrow_forwardThe 1H NMR spectrum of 1,2-dimethoxyethane (CH3OCH2CH2OCH3) recorded on a 300 MHz NMR spectrometer consists of signals at 1017 Hz and 1065 Hz downeld from TMS. (a) Calculate the chemical shift of each absorption. (b) At what frequency would each absorption occur if the spectrum were recorded on a 500 MHz NMR spectrometer?arrow_forwardDraw an isomer of dichlorocyclopropane that gives an 1H NMR spectrum a. with one signal. b. with two signals. c. with three signals.arrow_forward
- From the spectra, determine the structure of the molecule.Remember to ignore the triad in the 13 C NMR spectrum at 77 ppm thatcomes from the NMR solventarrow_forwardThis is the 13C spectrum for an X compound with molecular formula C7H12O4. The substance is not soluble in NaHCO3 and has a stretch at 1740cm-1 on the IR spectrum. What is the structure of X?arrow_forwardThe 1H NMR spectrum of 1,2-dimethoxyethane (CH3OCH2CH2OCH3) recorded on a 300 MHz NMR spectrometer consists of signals at 1017 Hz and 1065 Hz downfield from TMS. (a) Calculate the chemical shift of each absorption. (b) At what frequency would each absorption occur if the spectrum were recorded on a 500 MHz NMR spectrometer?arrow_forward
- Give An introduction to NMR spectroscopy ?arrow_forwardMatch each compound with the appropriate IR spectrum.arrow_forwardConsider isomeric alcohols A and B and mass spectra [1] and [2]. (a) Label the molecular ion and base peak in each spectrum. (b) Use thefragmentation patterns to determine which mass spectrum correspondsto isomer A and which corresponds to isomer B.arrow_forward
- Please explain the differences in ir spectra for an (EtOH) alcohol in a liquid phase and in a gas phase.arrow_forwardConsider isomeric alcohols A and B and mass spectra [1] and [2].(a) Label the molecular ion and base peak in each spectrum. (b) Use the fragmentation patterns to determine which mass spectrum corresponds to isomer A and which corresponds to isomer B.arrow_forwardLable the peaks for this Benzophenone IR spectra and explainarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

