
Concept explainers
To explain: The following,
'a'If isomaltose is a mono-, di-, or polysaccharide?
Answer to Problem 13.55UTC
Solution:
'a'Isomaltose is a disaccharide.
Given: Structure of isomaltose in Haworth projection
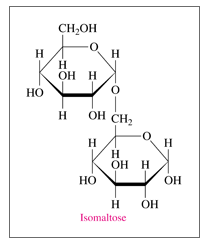
a.
Explanation of Solution
Isomaltose is a disaccharide as it contains two monosaccharides i.e.sugar units which are obtained after hydrolysis.
Therefore isomaltose is a disaccharide
b. The monosaccharides in isomaltose are two glucose units.
c. There is C1-C6 (a-a) O-glycosidic link in isomaltose.
Glycosidic link is the bond which connects two monosaccharides through anomeric carbon (a carbon which is coming from carbonyl group and attached with two oxygen atoms). In case of isomaltose, the anomeric carbon i.e. hemiacetal is linked with another sugar moiety through O atom. Thus this is a C1-C6 (a-a) O-glycosidic link.
Therefore, the monosaccharides in isomaltose are two glucose units and there is C1-C6 O-glycosidic link.
d. The given structure is an a-isomer of isomaltose.
As the OH group in the anomeric carbon is downward i.e. below the plane, so it is a-anomer (a-isomer of isomaltose).
Therefore the given structure is an a-isomer of isomaltose
e. Isomaltose is a reducing sugar
Isomaltose is a reducing sugar as it is capable of reducing Tollen’s reagent and Fehling solution. All the monosaccharides (aldoses) are reducing sugars along with some disaccharides. A disaccharide (as for example isomaltose) which has one anomeric carbon involved in glycosidic linkage but other anomeric carbon is free then it is able to reduce Tollen’s and Fehling solution.
Thus, isomaltose though a disaccharide is a reducing sugar.
- Isomaltose is a disaccharide.
- The monosaccharides in isomaltose are glucose units.
- The glycosidic link in isomaltose is C1-C6 (a-a) O-glycosidic link.
- The given structure is an a-isomer of isomaltose
- Isomaltose is a reducing sugar.
Want to see more full solutions like this?
Chapter 13 Solutions
Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





