
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 14PS
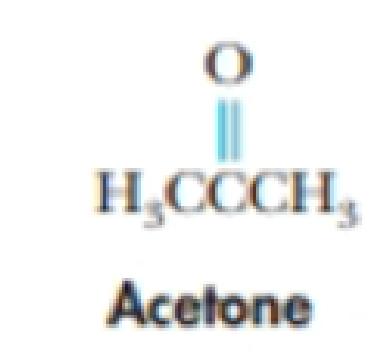
Acetone, CH3COCH3, is quite soluble in water. Explain why this should be so.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Explain the molecular reason why the vapor pressure decreases when glycerol (C3H5(OH)3) is dissolved in the solvent
Explain why cholesterol, a compound with molecular formula C 27H 46O and one OH group, is soluble in CCl 4 but insoluble in water.
Explain why many alcohols are soluble in water. What part of an alcohol will limit its solubility in water? What part of an alcohol may make it soluble in water?
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Ch. 13.1 - (a) If you dissolve 10.0 g (about one heaping...Ch. 13.2 - Use the data in Table 13.1 to calculate the...Ch. 13.3 - Prob. 13.3CYUCh. 13.4 - Assume you dissolve 10.0 g of sucrose (C12H22O11)...Ch. 13.4 - What quantity of ethylene glycol, HOCH2CH2OH, must...Ch. 13.4 - In the northern United States, summer cottages are...Ch. 13.4 - Bradykinin is a small peptide (9 amino acids; 1060...Ch. 13.4 - An aluminum-containing compound has the empirical...Ch. 13.4 - A 1.40-g sample of polyethylene, a common plastic,...Ch. 13.4 - Calculate the freezing point of 525 g of water...
Ch. 13.5 - The blue line on the diagram illustrates the...Ch. 13.5 - How many theoretical plates are required to...Ch. 13.5 - Prob. 1.3ACPCh. 13.5 - The vapor pressure of pure heptane is 361.5 mm Hg...Ch. 13.5 - If the headspace of a soda is 25 mL and the...Ch. 13.5 - Prob. 2.2ACPCh. 13.5 - Prob. 2.3ACPCh. 13.5 - Prob. 2.4ACPCh. 13.5 - Prob. 3.1ACPCh. 13.5 - Prob. 3.2ACPCh. 13.5 - Prob. 3.3ACPCh. 13 - You dissolve 2.56 g of succinic acid, C2H4(CO2H)2,...Ch. 13 - You dissolve 45.0 g of camphor, C10H16O, in 425 mL...Ch. 13 - Prob. 3PSCh. 13 - Prob. 4PSCh. 13 - Prob. 5PSCh. 13 - Prob. 6PSCh. 13 - Prob. 7PSCh. 13 - Prob. 8PSCh. 13 - Hydrochloric acid is sold as a concentrated...Ch. 13 - Concentrated sulfuric acid has a density of 1.84...Ch. 13 - The average lithium ion concentration in seawater...Ch. 13 - Silver ion has an average concentration of 28 ppb...Ch. 13 - Which pairs of liquids will be miscible? (a) H2O...Ch. 13 - Acetone, CH3COCH3, is quite soluble in water....Ch. 13 - Prob. 15PSCh. 13 - Use the following data to calculate the enthalpy...Ch. 13 - You make a saturated solution of NaCl at 25 C. No...Ch. 13 - Some lithium chloride, LiCl, is dissolved in 100...Ch. 13 - Prob. 19PSCh. 13 - The Henrys law constant for O2 in water at 25 is...Ch. 13 - An unopened soda can has an aqueous CO2...Ch. 13 - Hydrogen gas has a Henrys law constant of 7.8 104...Ch. 13 - A sealed flask contains water and oxygen gas at 25...Ch. 13 - Butane, C4H10, has been suggested as the...Ch. 13 - A 35.0-g sample of ethylene glycol, HOCH2CH2OH, is...Ch. 13 - Urea, (NH2)2CO, which is widely used in...Ch. 13 - Pure ethylene glycol, HOCH2CH2OH, is added 2.00 kg...Ch. 13 - Pure iodine (105 g) is dissolved in 325 g of CCl4...Ch. 13 - Prob. 29PSCh. 13 - What is the boiling point of a solution composed...Ch. 13 - Prob. 31PSCh. 13 - Prob. 32PSCh. 13 - Prob. 33PSCh. 13 - Some ethylene glycol, HOCH2CH2OH, is added to your...Ch. 13 - You dissolve 15.0 g of sucrose, C12H22O11, in a...Ch. 13 - A typical bottle of wine consists of an 11%...Ch. 13 - Prob. 37PSCh. 13 - Estimate the osmotic pressure of human blood at 37...Ch. 13 - An aqueous solution containing 1.00 g of bovine...Ch. 13 - Calculate the osmotic pressure of a 0.0120 M...Ch. 13 - You add 0.255 g of an orange, crystalline compound...Ch. 13 - Butylated hydroxyanisole (BHA) is used in...Ch. 13 - Benzyl acetate is one of the active components of...Ch. 13 - Anthracene, a hydrocarbon obtained from coal, has...Ch. 13 - An aqueous solution contains 0.180 g of an...Ch. 13 - Aluminon, an organic compound, is used as a...Ch. 13 - Prob. 47PSCh. 13 - To make homemade ice cream, you cool the milk and...Ch. 13 - List the following aqueous solutions in order of...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - When solutions of BaCl2 and Na2SO4 are mixed, the...Ch. 13 - The dispersed phase of a certain colloidal...Ch. 13 - Phenylcarbinol is used in nasal sprays as a...Ch. 13 - (a) Which aqueous solution is expected to have the...Ch. 13 - Arrange the following aqueous solutions in order...Ch. 13 - Prob. 56GQCh. 13 - Dimethylglyoxime [DMG, (CH3CNOH)2] is used as a...Ch. 13 - A 10.7 m solution of NaOH has a density of 1.33...Ch. 13 - Concentrated aqueous ammonia has a molarity of...Ch. 13 - Prob. 60GQCh. 13 - If you want a solution that is 0.100 m in ions,...Ch. 13 - Consider the following aqueous solutions: (i) 0.20...Ch. 13 - (a) Which solution is expected to have the higher...Ch. 13 - The solubility of NaCl in water at 100 C is 39.1...Ch. 13 - Instead of using NaCl to melt the ice on your...Ch. 13 - The smell of ripe raspberries is due to...Ch. 13 - Hexachlorophene has been used in germicidal soap....Ch. 13 - The solubility of ammonium formate, NH4CHO2, in...Ch. 13 - How much N2 can dissolve in water at 25 C if the...Ch. 13 - Cigars are best stored in a humidor at 18 C and...Ch. 13 - An aqueous solution containing 10.0 g of starch...Ch. 13 - Prob. 72GQCh. 13 - Calculate the enthalpies of solution for Li2SO4...Ch. 13 - Water at 25 C has a density of 0.997 g/cm3....Ch. 13 - If a volatile solute is added to a volatile...Ch. 13 - A solution is made by adding 50.0 mL of ethanol...Ch. 13 - A 2.0% (by mass) aqueous solution of novocainium...Ch. 13 - A solution is 4.00% (by mass) maltose and 96.00%...Ch. 13 - The following table lists the concentrations of...Ch. 13 - A tree is 10.0 m tall. (a) What must be the total...Ch. 13 - Prob. 81GQCh. 13 - A compound is known to be a potassium halide, KX....Ch. 13 - Prob. 85GQCh. 13 - If one is very careful, it is possible to float a...Ch. 13 - A solution of benzoic acid in benzene has a...Ch. 13 - You dissolve 5.0 mg of iodine, I2, in 25 mL of...Ch. 13 - Prob. 89ILCh. 13 - In a police forensics lab, you examine a package...Ch. 13 - An organic compound contains carbon (71.17%),...Ch. 13 - Prob. 92ILCh. 13 - When sails of Mg2+, Ca2+, and Be2+ are placed in...Ch. 13 - Explain why a cucumber shrivels up when it is...Ch. 13 - Prob. 95SCQCh. 13 - A 100.-gram sample of sodium chloride (NaCl) is...Ch. 13 - Prob. 97SCQCh. 13 - Prob. 98SCQCh. 13 - Starch contains CC, CH, CO, and OH bonds....Ch. 13 - Prob. 100SCQCh. 13 - You have two aqueous solutions separated by a...Ch. 13 - Prob. 102SCQCh. 13 - Sodium chloride (NaCl) is commonly used to melt...Ch. 13 - Prob. 105SCQCh. 13 - Prob. 106SCQCh. 13 - Prob. 107SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Draw a Lewis structure for each of the following species: a. H2CO3 b. CO32 c. CH2O d. CO2
Essential Organic Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 76. Space-filling diagrams for the structures of vitamin A, vitamin D, and vitamin C are shown below. Which are fat soluble? Which are water soluble? What is the difference between the structures of water-soluble and fat-soluble vitamins?arrow_forwardAccount for these facts: (a) Although ethanol (C2H5OH) (bp, 80 C) has a higher molar mass than water (bp, 100 C), the alcohol has a lower boiling point. (b) Mixing 50 mL of ethanol with 50 mL of water produces a solution with a volume slightly less than 100 mL.arrow_forwardHow could the data in Table 13.2 be used to predict the solubility in water of 1-octanol or 1-decanol? Table 13.2 Solubilities of Alcohols in Water, 20°Carrow_forward
- Consider this information regarding two compounds. Thallium azide: yellow crystalline solid; melting point = 330 C; slightly soluble in water, more soluble in hot water; insoluble in ethanol or diethyl ether. Camphene: colorless, cubic crystals; melting point = 51 C; boiling point = 159 C; insoluble in water; moderately soluble in ethanol; soluble in diethyl ether. (a) Is camphene an ionic or molecular compound? Explain your answer. (b) Is thallium azide an ionic or molecular compound? Explain your answer.arrow_forwardIs lactose polor? Is lactose soluble in H2O? Are triglycerides Polar? Are triglycerides soluble in H20? Are small proteins polar? Are small proteins soluble in H20? Are large proteins polar? Are large proteins soluble in H20? Methylene chloride ("proper" IUPAC name= dichloromethane) is considered a nonpolar solvent. Which of the four types of compounds listed above should dissolve in methylene chloride? Explainarrow_forwardA state of dynamic equilibrium exists between the solid sucrose (table sugar) molecules, C12H22O11, and sucrose molecules dissolved in aqueous solution for a saturated sucrose solution. a. Write the equation to represent the dynamic equilibrium. b. What change occurs in the concentration of dissolved sucrose if more solid sucrose is added to the saturated system? Explain. c. If water is added to the saturated solution and the equilibrium is reestablished, what change occurs to the amount (moles) of dissolved sucrose? Explain. d. If water is added to the saturated solution and the equilibrium is reestablished, what change occurs in the concentration (molarity) of dissolved sucrose? Explain.arrow_forward
- What solute particles are present in an aqueous solution of CH3COCH3?arrow_forwardWhat is the osmotic pressure of a 4.5 L solution containing 30.6 g of fructose (C6H12O6 at 40 °C? (Step by Step)arrow_forwardWhat is the osmotic pressure of a 4.5 FL OZ solution containing 30.6 g of fructose (C6H12O6) at 40 °F. Osmotic pressure is 29.2 atm. Explain why.arrow_forward
- What is the osmotic pressure of a solution prepared by adding 12.5 g of sucrose (C12H22O11) to enough water to make 450 mL of solution at 25 °C?arrow_forwardExplain in 1 sentence why sugar is soluble in water in terms of their molecular interactions.arrow_forwardOsmotic pressure may be used to determine the molecular weight of pure substances. It is an especially useful technique because low concentrations of solute produce relatively large osmotic pressures. Although more sophisticated techniques are now available to determine molecular weight, osmotic pressure is still used occasionally because of its simplicity. Determine the molecular weight of myoglobin (an oxygen storage protein that gives muscle its red color). The osmotic pressure of a 1.0-mL water solution containing 1.5 x 10 ~3 g of the protein was measured at 2.06 x 10 ~3 atm at 25°C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry In Focus
Chemistry
ISBN:9781305084476
Author:Tro, Nivaldo J., Neu, Don.
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY