
Concept explainers
Determine what state this substance is in at 1 atm and 298 K by referring to its phase diagram.
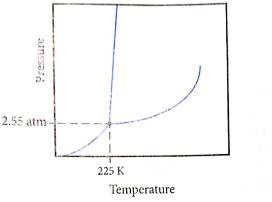
a) Solid
b) Li quid
c) Gas
d) All three states in equilibrium
Interpretation: The state of a substance at
Concept introduction: A phase diagram represents what phases exist at equilibrium and the phase transformation that one can expect on changing one of the parameters of the system
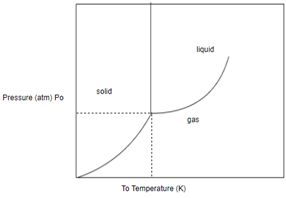
In order to determine the phase of a substance, draw a horizontal line corresponding to given pressure and a vertical line corresponding to given temperature. The point of intersection of these two horizontal and vertical lines gives the idea in which phase the given material exist.
To determine: what state this substance is at
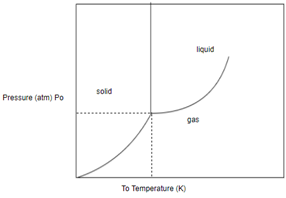
Answer to Problem 1SAQ
Solution: The phase of this substance at
Explanation of Solution
Given, Pressure
Temperature
And following phase diagram
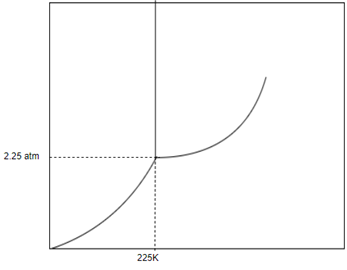
Draw horizontal and vertical line corresponding to given temperature and pressure.
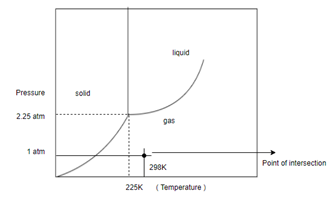
From above diagram it becomes clear that point of intersection is in gas phase so this substance at
Phase of any substance is function of both temperature and pressure.
Want to see more full solutions like this?
Chapter 13 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: Structure and Properties
- If you've ever opened a bottle of rubbing alcohol or other solvent on a warm day, you may have heard a little “whoosh” as the vapor that had built up above the liquid escapes. Describe on a microscopic basis how a vapor pressure builds up in a closed container above a liquid. What processes in the container give rise to this phenomenon?arrow_forwardGiven the following data about xenon, normalboilingpoint=108Cnormalmeltingpoint=112Ctriplepoint=121Cat281mmHgcriticalpoint=16.6Cat58atm (a) Construct an approximate phase diagram for xenon. (b) Estimate the vapor pressure of xenon at -115C. (c) Is the density of solid Xe larger than that for liquid Xe?arrow_forwardThe molar heat of vaporization of substance X is 34kJ/mol; of substance Y, 27kJ/mol. Which substance would be expected to have the higher normal boiling point? The higher vapor pressure at 25C?arrow_forward
- Consider the following data for xenon: Triple point: 121C, 280 torr Normal melting point: 112C Normal boiling point: 107C Which is more dense, Xe(s) or Xe(l)? How do the melting point and boiling point of xenon depend on pressure?arrow_forwardWhat feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container?arrow_forwardCarbon tetrachloride, CCl4, was once used as a dry cleaning solvent, but is no longer used because it is carcinogenic. At 57.8 C, the vapor pressure of CCl4 is 54.0 kPa, and its enthalpy of vaporization is 33.05 kJ/mol. Use this information to estimate the normal boiling point for CC14.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





