
Concept explainers
Draw the enol tautomers for each of the following compounds. If the compound has more than one enol tautomer, indicate which one is more stable.
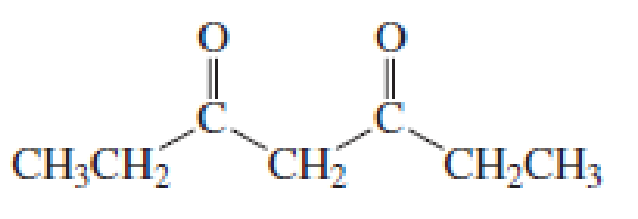
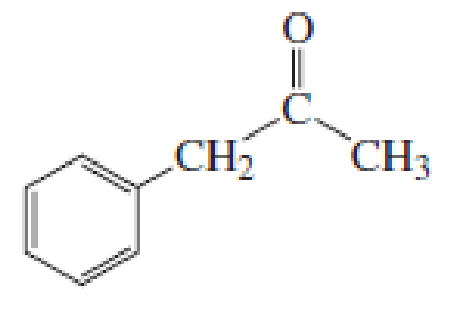
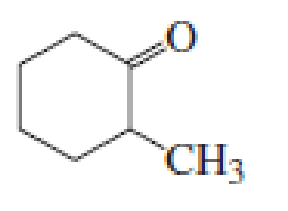
(a)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
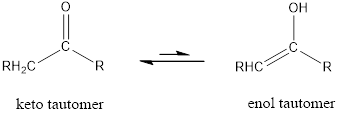
Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
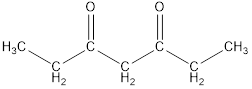
The only difference in keto-enol tautomer is the location of hydrogen and double bond.
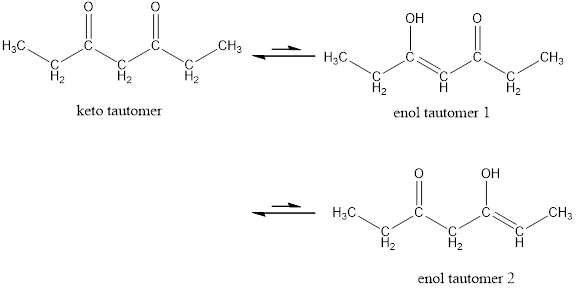
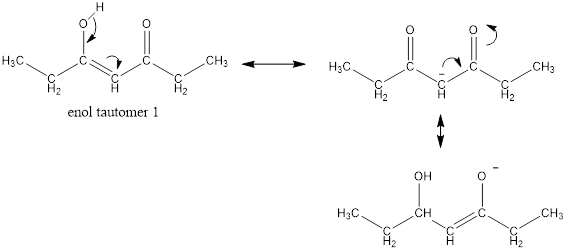
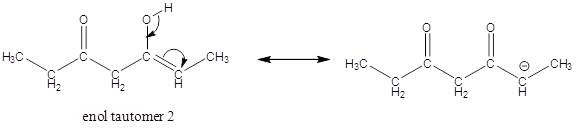
Enol tautomer 1 is more stable than enol tautomer 2.
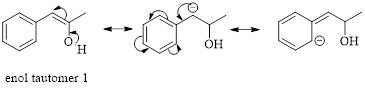
Enol tautomer 1 can undergo delocalization and are more stable.
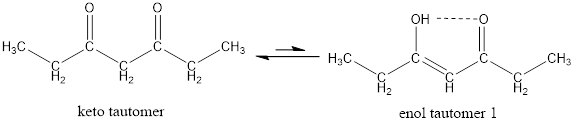
Thus enol tautomer 1 is more stable since it has more resonance structures and also possess intramolecular hydrogen bonding.
(b)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
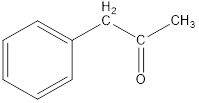
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
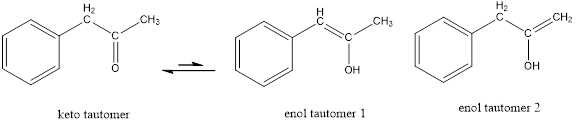
These tautomers undergo resonance and are shown below,
Enol tautomer 1 can undergo delocalization. Enol tautomer 1 is more stable than enol tautomer 2.
The enol tautomer 1 is more stable because there is a conjugation between the double bond and benzene ring. No such conjugation is possible in the enol tautomer 2.
(c)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Explanation of Solution
Given keto tautomer is,
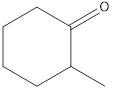
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
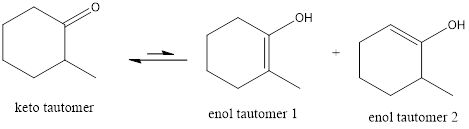

The alkene double bond formed by tautomer 1 is tetra-substituted which is stable than tautomer 2.
Hence, enol tautomer 1 is more stable.
Want to see more full solutions like this?
Chapter 13 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw the structures of the initially formed enol tautomers in the reactions of propyne and dicyclohexylethyne with dicyclohexylborane followed by NaOHNaOH and H2O2H2O2arrow_forwardDraw the keto form of the following enol.arrow_forwardDrawing an Enol and a Ketone Formed by Hydration of an Alkyne Draw the enol intermediate and the ketone product formed in the following reaction.arrow_forward
- Choose the most correct set of reagents for the following reaction.arrow_forwardExplain why the following bromoketone forms different bicyclic compounds under different reaction conditions:arrow_forwardWhat is the product resulted due to the reaction of 2-hydroxy-3-methoxybenzaldehyde with ethyl bromoacetate in a basic solution indicating all the reagents and intermediates occurred during the reaction? What is the product obtained when product A reacted with ethyl acetate in the same basic solution?arrow_forward
- Chose the correct reagent that will accomplish the following transformations:arrow_forwardDraw the enol form of each keto tautomer in parts (a) and (b), and the keto form of each enol tautomer in parts (c) and (d).arrow_forwardRank the compounds in each group in order of increasing reactivity in nucleophilic acyl substitution. C6H5CO2CH3, C6H5COCl, C6H5CONH2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





