
Concept explainers
In the mass spectrum of the following compounds, which is the tallest—the peak at m/z =57 or the peak at m/z = 71?
- a. 3·methylpentane
- b. 2·methylpentnne
Interpretation
The tallest peak should be identified.
Explanation of Solution
Mass fragmentation of 3-methyl pentane: In this molecule will be more adopted to lose an ethyl radical forming a secondary carbocation and a primary radical than a methyl radical forming secondary carbocation and methyl radical. In addition, 3-methylpentane ha undergoes for two path ways to lose an ethyl radical,
Therefore, the peak at
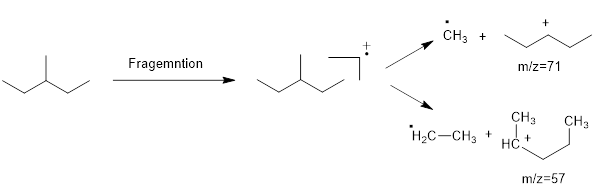
Mass fragmentation of 2-methyl pentane: The 2-methylpentane has undergoes for pathways to lose a methyl radical in each pathway and it cannot form a secondary carbocation by losing an ethyl radical. Loss of an ethyl radical would form a primary carbocation and a primary radical. Therefore, it will be more adopt to lose a methyl radical than an ethyl radical, the corresponding peak at
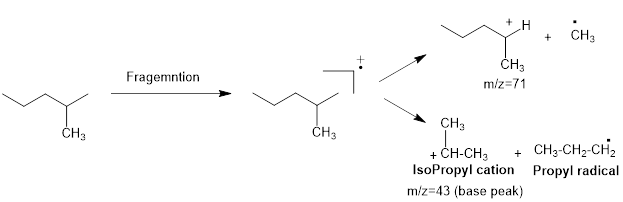
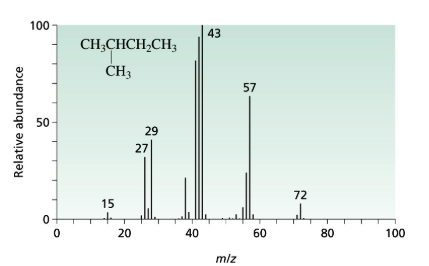
The 3-methyl pentane molecular peak at the m/e=71 has more intense then the peak at
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY
- In the mass spectrum of the following compounds, which is the tallest—the peak at m/z = 57 or the peak at m/z = 71? a. 3-methylpentane b. 2-methylpentanearrow_forwardMass spectrometry is often used to both identify and quantify compounds. What is different between mass spectra with changing concentrations of a compound? How would you design a method (i.e. what would you need) to both identify and quantify a compound using mass spectrometry?arrow_forward1. Mass spectrometry will give information about molecular mass, chemical formula and structure of the molecule. Select one: True False 2. The strongest peak with 100% abundance in the mass spectrum is known as ........................................... a.Prominent ion peak b.Fragment ion peak c.Molecular ion peak d.Base peakarrow_forward
- How to determine the 2 molecular formulas, one without oxygens and one with 1 or 2 oxygens from a mass spectrometry spectrum.arrow_forwardMass spectroscopic. What is the isotopic peak pattern of each : Organic compound with 2 bromine atoms. Organic compound with 2 chlorine atoms.arrow_forwardIdentify two peaks that are expected to appear in the mass spectrum of 3-pentanol. For each peak, identify the fragment associated with the peak.arrow_forward
- A) Label the mass spectrum of 1-butanol,3-methyl (Be sure to write the chemical formula for each major peak) (major peaks include 88, 70, 55, 42, and 43 m/z)arrow_forwardCan you help me analyse these spectrum tests? The unknown compound has a BP of 102, %C is 68.54, and %H is 8.66. The given tests are Mass Spec. and Thin-Layer Chromatography.arrow_forwardIdentify the fragments of 1-bromohexane in the mass spectrumarrow_forward
- In a mass spectrum, the peak of greatest abundance is referred to as the_________________________. Relative abundance is the unit along the y-axis ina mass spectrum. What are the units on the x-axis? _----------------------------arrow_forwardAfter creating her standard curve for absorption versus the concentration (M) of FD&C Red 40, a student found that her best fit linear line for FD&C Red 40 was y = 2,962x + 0.005. Her Kool-Aid sample had an absorbance of 0.685. If 0.543 grams of Kool-Aid powder was used to prepare an 8-fl oz cup of her assigned flavor, what is the percent by mass of FD&C Red 40 in her 8-fl oz cup?arrow_forwardHow many carbon atoms are expected to be in a hydrocarbon sample if it shows M and M+1 peak intensities of 67.8 and 6.04 respectively?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





