
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 51E
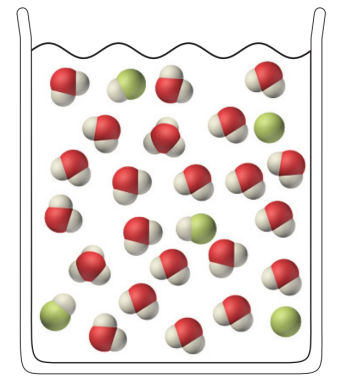
Determine from the following molecular view of a hydrofluoric acid (HF) solution whether HF is a strong or a weak acid:
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 13 Solutions
Chemistry In Focus
Ch. 13 - Which property is not generally associated with...Ch. 13 - Prob. 2SCCh. 13 - The ideal pH of a swimming pool is 7.2. You...Ch. 13 - Prob. 13.1YTCh. 13 - Identify the Brnsted-Lowry acid and base in the...Ch. 13 - Prob. 1ECh. 13 - What are the properties of acids?Ch. 13 - Prob. 3ECh. 13 - Prob. 4ECh. 13 - List five common laboratory acids and their uses.
Ch. 13 - Why are bases not commonly found in foods?Ch. 13 - List four common laboratory bases and their uses.Ch. 13 - What are the Arrhenius definitions of acids and...Ch. 13 - What are the Brnsted-Lowry definitions of acids...Ch. 13 - What is the difference between a strong acid and a...Ch. 13 - Prob. 11ECh. 13 - What pH range is considered acidic? Basic?...Ch. 13 - What acid is responsible for the sour taste of...Ch. 13 - What is pickling? What acid is responsible for the...Ch. 13 - Prob. 15ECh. 13 - Prob. 16ECh. 13 - List several common acids and where they might be...Ch. 13 - Prob. 18ECh. 13 - Prob. 19ECh. 13 - What causes acid indigestion? List some common...Ch. 13 - Prob. 21ECh. 13 - Explain how a leavening agent works.Ch. 13 - Prob. 23ECh. 13 - Prob. 24ECh. 13 - Prob. 25ECh. 13 - Prob. 26ECh. 13 - Prob. 27ECh. 13 - Prob. 28ECh. 13 - Write a chemical equation to show the...Ch. 13 - Write a chemical equation to show the...Ch. 13 - Identify the Brnsted-Lowry acid and base in each...Ch. 13 - Identify the Brnsted-Lowry acid and base in each...Ch. 13 - Write a chemical equation using Lewis structures...Ch. 13 - Write a chemical equation using Lewis structures...Ch. 13 - A chemist makes two solutions. One is a 0.01-MHCl...Ch. 13 - A chemist makes a 0.001-MNaOH solution and a...Ch. 13 - Give the pH that corresponds to each solution and...Ch. 13 - Give the pH that corresponds to each solution and...Ch. 13 - What is the [H3O+] in a solution with a pH of 4?Ch. 13 - What is the [H3O+] in a solution with a pH of 11?Ch. 13 - Write chemical reactions to show how each antacid...Ch. 13 - Write chemical reactions to show how each antacid...Ch. 13 - Suppose that the stomach contains...Ch. 13 - Suppose that 250.0 mL of a basic solution is 0.100...Ch. 13 - Prob. 45ECh. 13 - Write a chemical reaction to show how NO2 forms...Ch. 13 - Prob. 47ECh. 13 - Prob. 50ECh. 13 - Determine from the following molecular view of a...Ch. 13 - Determine from the following molecular view of a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why equilibrium arrows are used in the ionization equations for some acids.arrow_forwardWrite equations to illustrate the acid-base reaction when each of the following pairs of Brnsted acids and bases are combined: Acid Base a.HOCl H2O b.HClO4 NH3 c.H2O NH2 d.H2O OCl e.HC2O4 H2Oarrow_forwardEach box represents an acid solution at equilibrium. Squares represent H+ ions, and circles represent the anion. Water molecules are not shown. Which figure represents a strong acid? Which figure is a weak acid?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY